As technology undergoes continuous evolution, the associated challenges with data management and security are also on the rise. With widespread digitization, regulatory bodies, such as the FDA have implemented measures to ensure accountability. It is within this dynamic landscape that ALCOA+ data integrity principles have emerged as a crucial framework for effectively managing data throughout its lifecycle.
This article extensively explores strategies for upholding ALCOA+ principles and maintaining data integrity, covering the entire spectrum from data generation to archival. Additionally, we introduce SCW.AI’s GMP-compliant solutions, shedding light on how we can assist you in ensuring data integrity and minimizing the risk of receiving warning letters and penalties from regulatory bodies.
What is ALCOA+ Data Integrity?
Data integrity refers to the accuracy, consistency, and reliability of a dataset. It entails preserving the data from start to finish, ensuring it remains unchanged. Data integrity ensures that a dataset is processed accurately, comprehensively, and reliably.
ALCOA stands for; Attributable, Legible, Contemporaneous, Original, Accurate and the + refers to additional principles which are Complete, Consistent, Enduring, Available.
What are the 9 ALCOA+ Principles?

1. Attributable
Each data record should be attributable to a specific person or system. This is crucial to ensure compliance with regulations and established processes.
Digital solutions make it easy to specify creator or recorder information for each entry. This allows tracking the source of events and information, facilitating accountability.
2. Legible
This means that data should be clearly visible for relevant parties, such as regulators or internal auditors, to accurately assess the information.
Digital solutions enhance the legibility of information through a clear interface design, appropriate font usage, and effective information organization methods which allows users to better understand and interpret complex data.
3. Contemporaneous
Data records should be created and updated at the time the event occurs. Any retrospective corrections or changes should be documented with a justification.
Digitalization can be helpful to improve this principle since it excels in recording events instantly,facilitating accurate and real-time information flow.
4. Original
Data records should be stored in their original form without any manipulation as preserving data without interference enhances its reliability.
Digital solutions possess a robust ability to preserve the original state of records. Supported by digital signatures, time-stamping, and similar methods, these solutions ensure the protection of information from manipulation.
5. Accurate
Data records should reflect the true state of affairs fully and accurately. Complete and reliable data aids in making accurate decisions.
Digital solutions ensure precise recording and presentation of information. Reliable data entry and accuracy are fundamental priorities in information management.
Note: The “+” in the “ALCOA+” emphasizes additional principles beyond the traditional ALCOA ones. Let’s briefly review these principles:
6. Complete
Data records must be comprehensive from start to finish. They should include all necessary information and no data should be missing or ambiguous.
The “Complete” aspect reflects the capability of digital solutions to provide comprehensive information. Each record is maintained with all necessary data, ensuring completeness.
7. Consistent
Data records should be maintained in a consistent format and adhere to standards. Inconsistency can negatively impact accuracy and reliability.
Digital solutions focus on presenting information consistently while adhering to standards for uniformity. Compliance and consistency play a crucial role in data analysis and interpretation.
8. Enduring
Data records should remain unchanged and enduring during their storage period. Changes over time should be traceable and verifiable.
The “Enduring” principle signifies the ability of digital solutions to preserve information over an extended period. Data is stored resiliently and enduringly against changing conditions.
9. Available
Data records should be accessible whenever needed. This is crucial to ensure accessibility during audits or reviews.
Digital solutions offer accessibility to information anytime, anywhere. This provides users with flexibility and swift access to information.
These principles form the basis of a set of data integrity guidelines and standards used in regulated industries such as pharmaceuticals, biotechnology, and others. ALCOA+ provides a framework, particularly in regulatory inspections and quality assurance processes, to maintain and demonstrate data integrity.
Digital solutions offer significant advantages to companies in adhering to the core principles outlined by ALCOA+. Accurate, complete, consistent, enduring, and available information management is crucial for the efficiency and transparency of business processes. Digital solutions ensure the precise recording, comprehensive presentation, consistent delivery, long-term preservation, and rapid accessibility of sensitive information.
As a result, companies can enhance operational excellence, strengthen reliability, and gain a competitive edge. Embracing Alcoa principles seamlessly, digital solutions play a leading role in information management, assisting businesses in effectively managing their information assets and confidently navigating the future.
Importance of Data Integrity for Pharmaceutical Industry
Data integrity plays a vital role within the pharmaceutical industry, ensuring the safety, efficacy, and quality of pharmaceutical products. This concept encompasses the accuracy, consistency, and reliability of data throughout its lifecycle, from research and development to manufacturing, distribution, and post-market surveillance.
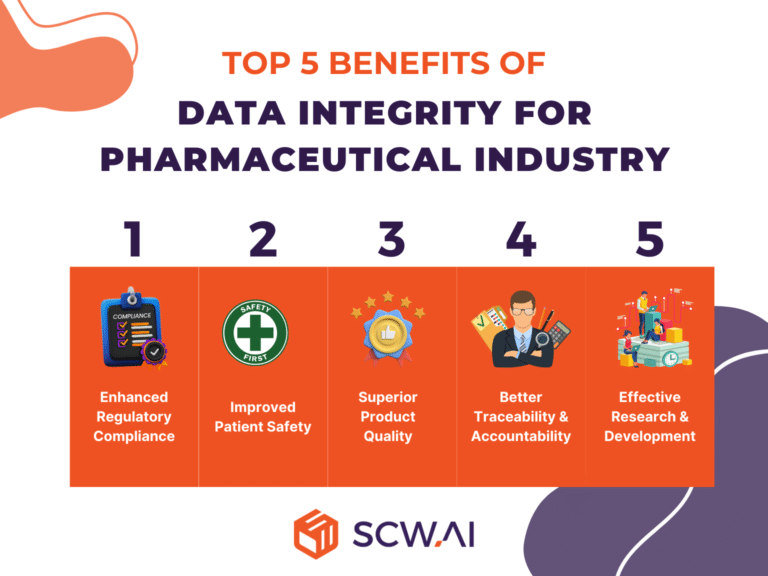
1. Regulatory Compliance
Data integrity is critical for regulatory compliance in the pharmaceutical industry. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose strict rules to ensure the integrity of data generated during drug development and manufacturing processes. Compliance with these rules is essential to obtain approvals and maintain licenses for pharmaceutical products.
2. Patient Safety
The pharmaceutical industry is required to provide safe and effective medications to patients. Data integrity ensures that information regarding the composition, manufacturing processes, and quality control of pharmaceutical products is accurate and reliable. This is crucial for preventing errors that could compromise patient safety.
3. Product Quality
Maintaining data integrity is directly linked to ensuring the quality of pharmaceutical products. Accurate and consistent data is essential for monitoring and controlling critical parameters throughout the manufacturing process. This guarantees that each batch of a pharmaceutical product meets the required quality standards.
4. Traceability and Accountability
Data integrity facilitates traceability, allowing companies to track and document every step of the pharmaceutical manufacturing process. This traceability not only ensures accountability but also provides a comprehensive overview of the product’s journey, from raw material sourcing to distribution.
5. Research and Development
In the early stages of drug development, data integrity is crucial for the validity and reliability of research findings. Accurate recording and reporting of experimental results are essential for making informed decisions about the progression of a drug candidate.
In conclusion, data integrity is a cornerstone of the pharmaceutical industry, contributing to regulatory compliance, patient safety, product quality, traceability, and the success of research and development efforts. Pharmaceutical companies must implement robust data management systems and practices to uphold the highest standards of integrity throughout their operations.
Ensuring Data Integrity with SCW.AI’s Paperless Quality Solutions
Our Paperless Manufacturing Solutions namely Digital Logbook and Digital Batch Record are dynamic solutions designed for our clients in the pharmaceutical industry. These solutions, which are fully compliant with ALCOA+ principles and current Good Manufacturing Practices (cGMP) standards, maximizes data integrity, ensuring continuous reliability. With attributable and traceable records, continuous monitoring, instant alerts, and user-friendly documentation and reporting features, Paperless Quality Solutions not only optimizes pharmaceutical manufacturing process documentation, including, logbooks, activity forms, batch records but also ensures readiness for regulatory audits.
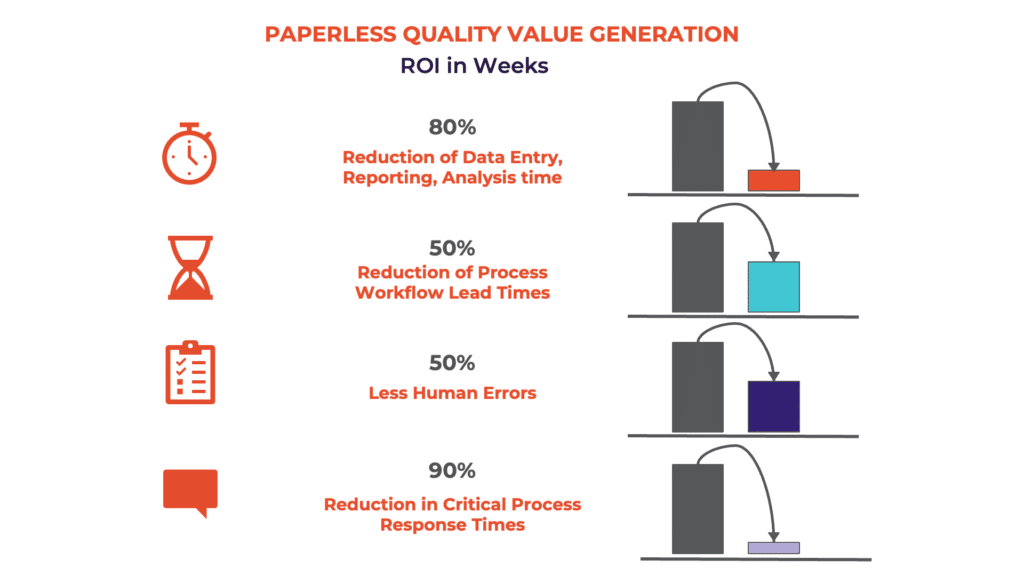
To discover SCW.AI’s Digital Logbook to strengthen your competitive advantage in the pharmaceutical industry and enhance data management effectiveness you can book a demo now and stand by for the second upcoming Paperless Quality Solution, the Digital Batch Record.