According to Mike Walker, Executive Director of Global Healthcare and Life Sciences Digital Strategy at Microsoft, less than 3% to 4% of pharmaceutical manufacturers meet the definition of being fully digital. Conversely, approximately 60% of pharma factories still rely on paper for data collection and storage, often feeding this information into outdated spreadsheets for analysis.
However, pharma 4.0 transformation is undeniably a priority investment. Data from PwC indicates that the majority of pharma manufacturers allocate 1.6% to 1.9% of their revenues toward developing the factory of the future. This committed investment underscores the critical nature of the transformation.
At the heart of this transition lies the most crucial strategic decision: selecting the right technological architecture. Manufacturers today face a complex choice among four primary models for their digital solutions:
- SaaS (Software as a Service)
- Low-Code platforms
- On-Premise
- In-House solutions.
Each model offers a distinct balance of control, cost structure, flexibility, time-to-value, and legacy cost burden. Therefore, a model suitable for one company might be a completely wrong choice for another. The wrong architectural decision can lead to prohibitive Total Cost of Ownership, slow deployment, and unnecessary complexities. The right foundation, however, unlocks sustainable scalability and positions the company for leadership in operational costs, productivity, and output.
This analysis provides a factual cost benefit analysis of these four paths. We will systematically examine each solution against the operational criteria specific to pharmaceutical production, helping IT and Operations leaders determine which foundation is best suited for their unique strategic goals in the pursuit of the modern Digital Factory.
Executive Summary
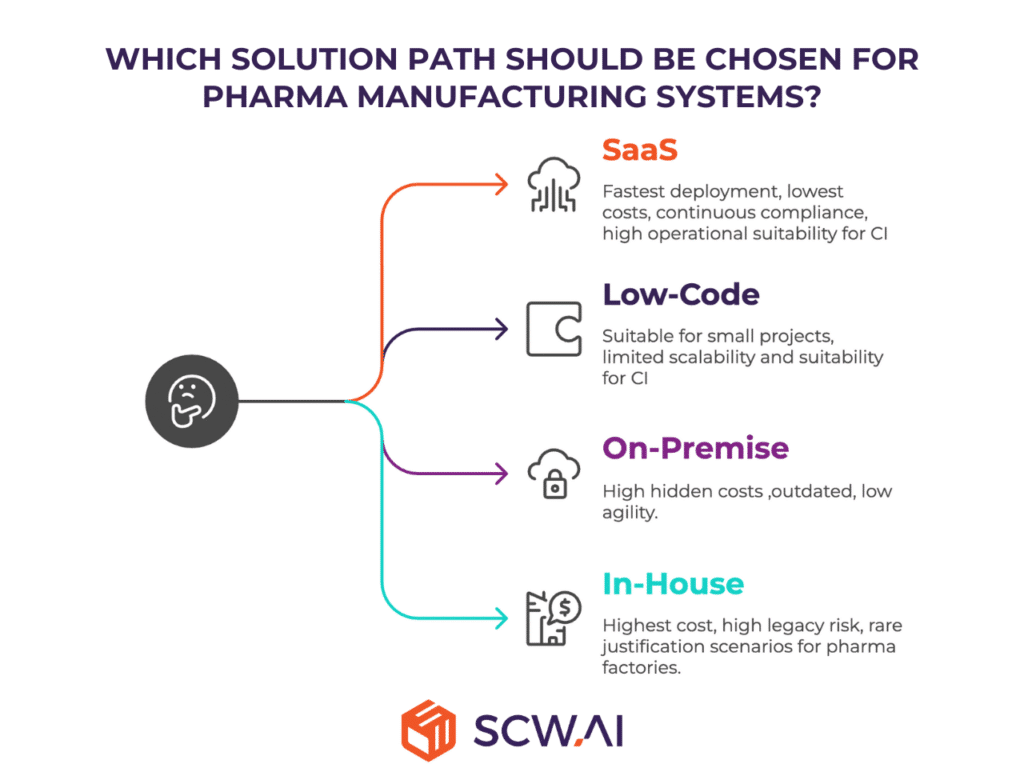
The analysis identifies four solution paths, each with distinct tradeoffs in efficiency, risk, and long-term sustainability:
- SaaS – Best overall fit for most pharma manufacturers. Enables fastest deployment, lowest labor and legacy costs, and continuous compliance. Leads in agility and efficiency.
- Low-Code – Suitable for small departmental projects, but not viable for enterprise-wide or continuous improvement systems. Limited to niche use cases.
- On-Premise – Fails to lead in any category. Outdated, costly, and low agility.
- In-House/Custom – Justified only in rare cases with proprietary manufacturing IP. Burdened by high talent costs and obsolescence risk. Highest cost, highest risk option.
Comparative Highlights
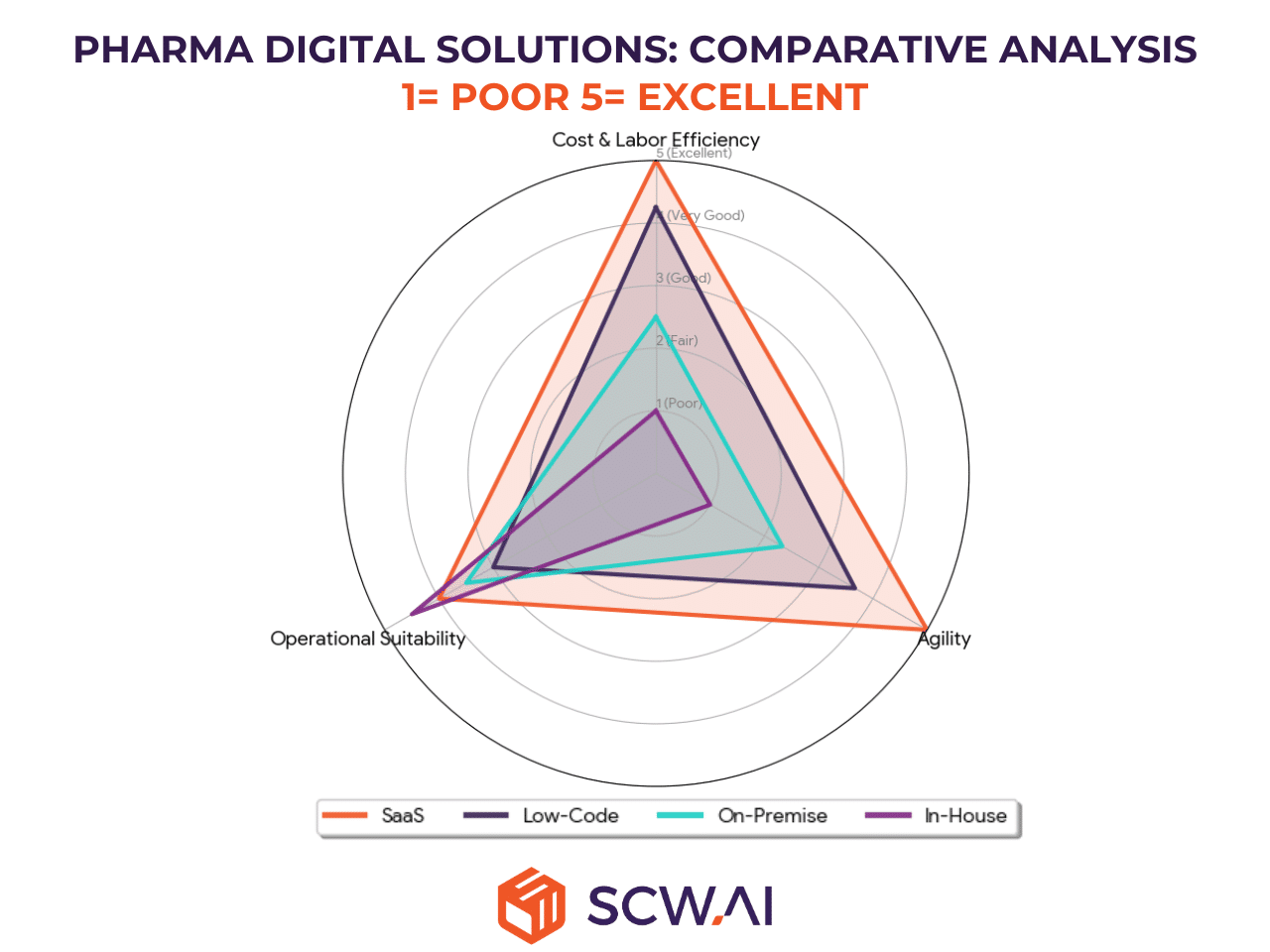
- Efficiency & Agility Leader: SaaS (shortest deployment, lowest labor burden, lowest legacy cost).
- Customization: In-House (perfect alignment but high costs).
Bottom Line
SaaS offers the strongest strategic fit and most sustainable path for the majority of pharma manufacturers. While In-House provides unmatched customization, it does so at the highest risk and cost. Low-Code remains a minor, localized tool, and On-Premise is a legacy choice with limited future relevance.
How We Compared Them: Key Criteria Driving Pharma Digital Strategy
To provide a factual cost benefit analysis across the four architectures, we utilized ten critical criteria grouped into three strategic dimensions. focusing on long-term operational value in pharma manufacturing.
1. Cost & Labor Efficiency Dimension
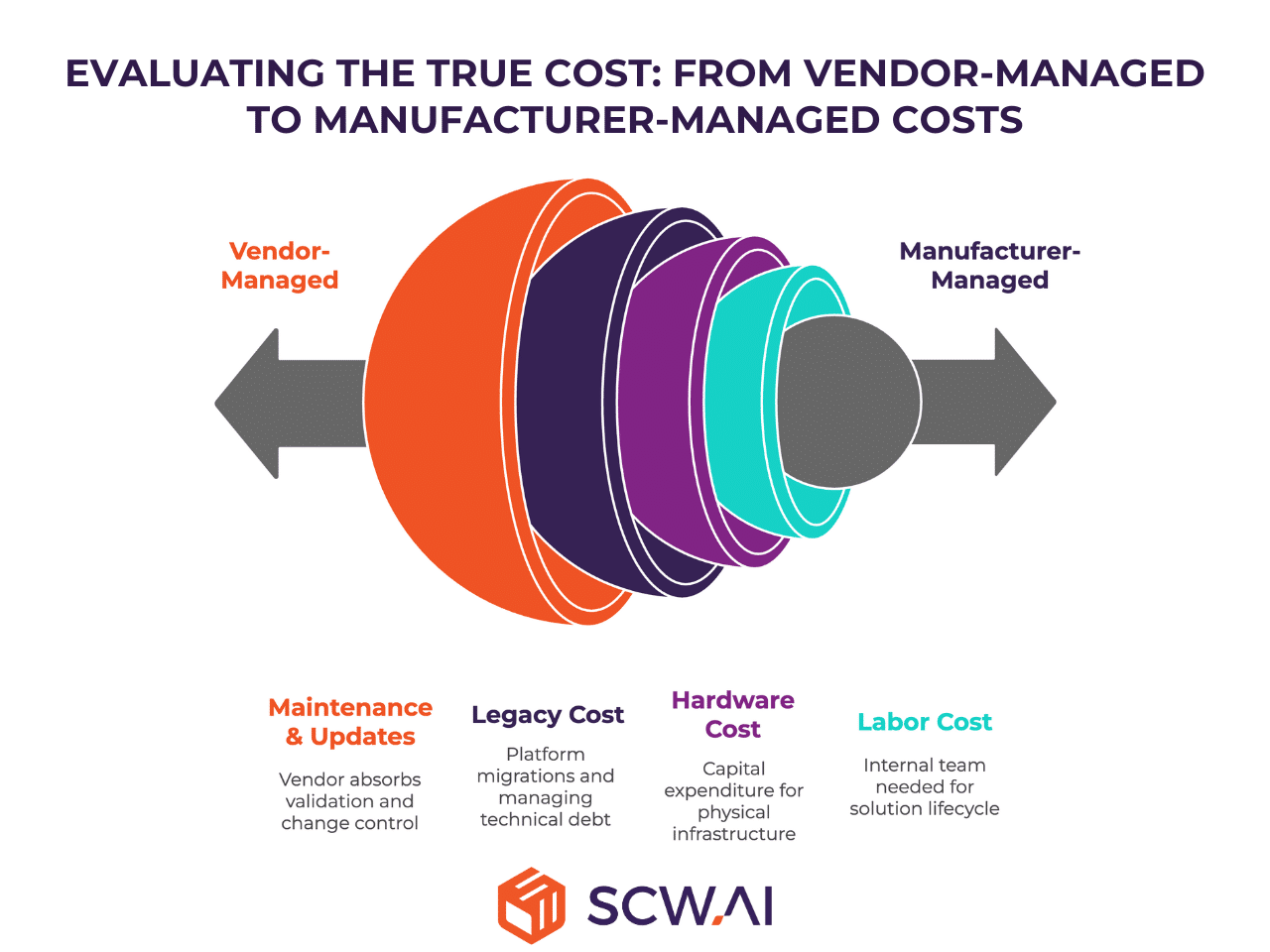
These factors quantify the ongoing financial and personnel burden the solution places on the manufacturer, determining the long-term economic sustainability of the platform.
- Labor Cost (Total): This measures the cost associated with the internal team needed to develop, design, implement, operate, and continuously validate the solution.
- Hardware Cost: This factor assesses the capital expenditure (CapEx) required for physical servers, networking equipment, storage, and disaster recovery infrastructure.
- Legacy Cost: It includes the expense of necessary platform migrations, managing technical debt, and securing obsolete systems. This cost accelerates dramatically if a platform lacks continuous vendor support.
- Maintenance & Updates & Bug Fix Costs/Efforts: It measures the effort (time, money, and validation resources) required to apply security patches, fix defects, and perform major system upgrades. Low effort means the vendor absorbs the majority of the continuous validation and change control work.
Agility Dimension
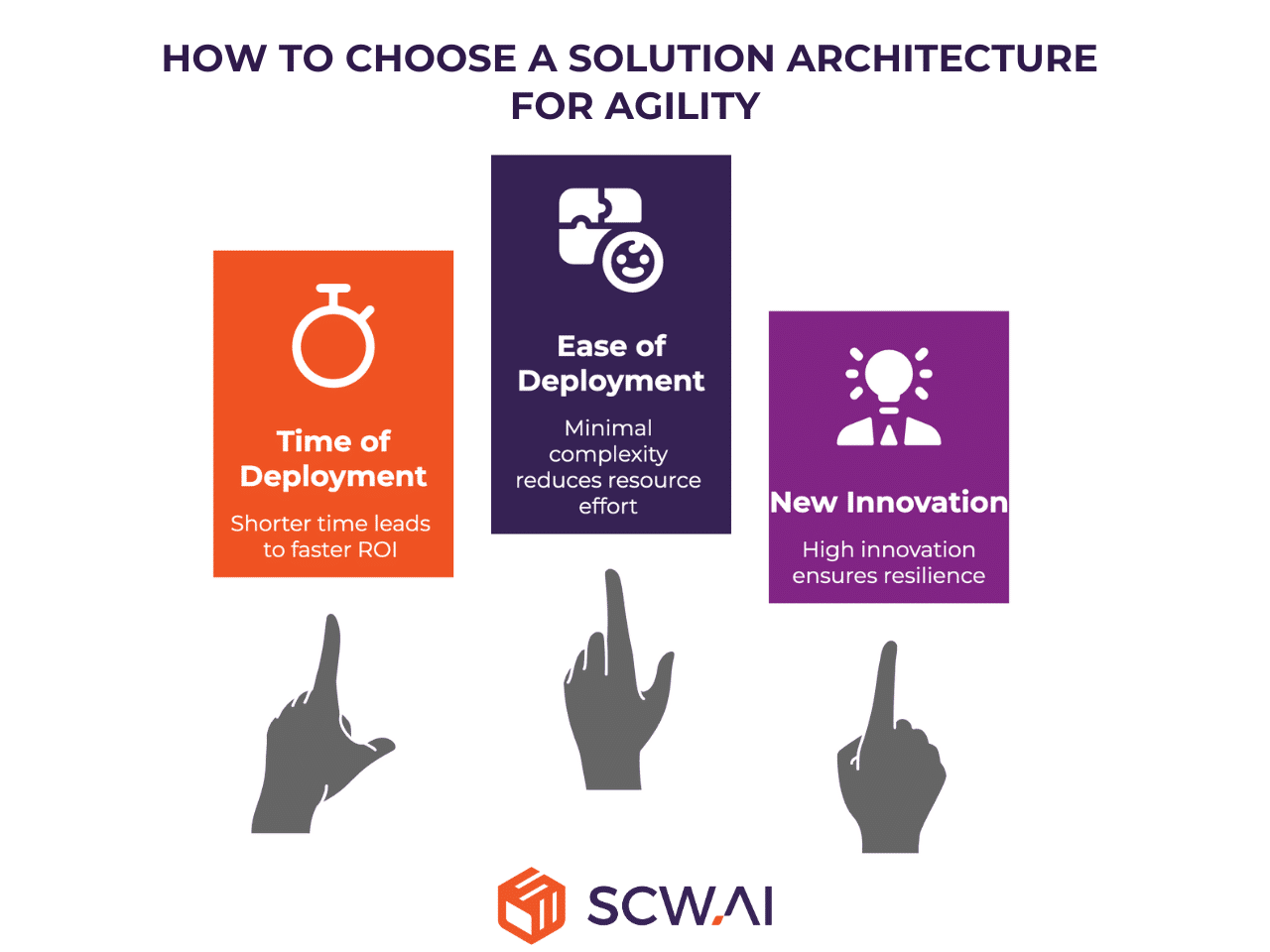
These factors assess how quickly the organization can deploy the solution and how effectively the system can adapt to future trends, market demands, and technological innovation.
- Time of Deployment: Measures the duration from project initiation to go-live. In pharma, a shorter time is crucial for faster ROI.
- Ease of Deployment: This evaluates the technical complexity and internal resource effort required to set up and configure the system. High ease typically reflects minimal custom coding and simplified, standardized installation procedures.
- New Features/Innovation: This assesses the rate at which new functionalities (e.g., AI capabilities, advanced Monitoring dashboards, new solution modules) are introduced and made available to the manufacturer. High rates of innovation provide greater resilience.
Operational Suitability Dimension
These factors address the core functionality, performance capacity, and security resilience needed for a mission-critical pharma manufacturing platform.
- Custom Performance: This measures the degree to which the software can be aligned with or optimized for the manufacturer’s unique and complex operational processes. High custom performance allows for deep integration and tailoring, often essential for proprietary workflows.
- Scalability: This measures the platform’s ability to efficiently and cost-effectively handle growth, whether it’s an increase in data volume, transaction speed, number of users, or the addition of new global manufacturing sites. Elastic scalability is paramount.
- Data Security: It evaluates the effectiveness of the security protocols, access controls, and data protection measures against cyber threats and unauthorized access, regardless of whether the platform is hosted internally or by a third party.
Detailed Analysis of SaaS, Low Code, On-Premise Cloud, In-House for Pharma Manufacturers
This section provides a detailed cost benefit analysis of the four digital architectures. For each model, we assess its performance across the ten critical criteria and define the specific type of pharma manufacturer for whom it is the ideal strategic fit.
1. SaaS (Software as a Service) Platforms
SaaS for Pharma involves subscribing to a specialized, often multi-tenant, Digital Factory platform that is hosted and fully managed by the vendor. In this model, the manufacturer accesses the software via the internet, which effectively shifts the heavy burden of infrastructure, security, and core application maintenance entirely to the provider.
Cost & Benefit Analysis
The SaaS model fundamentally changes the financial and labor equation, offering significant long-term advantages for the majority of manufacturers:
- Labor & Maintenance Cost: These costs are the lowest because internal IT teams are relieved of the need to develop software, purchase and manage servers, patch operating systems, and deploy major upgrades. All Maintenance & Updates are handled by the vendor. Consequently, there is no need to hire and retain personnel solely responsible for the healthy functioning, upkeep, and improvement of the core pharma manufacturing software.
- Scalability: SaaS provides unmatched Scalability. Manufacturers can instantly scale usage, provision new production lines, or add an entire new manufacturing site simply by updating their subscription. Crucially, there is no capital expenditure (CapEx) required for new hardware.
- Time & Ease of Deployment: SaaS offers the shortest time of deployment and highest ease of deployment because vendors often provide pre-configured solutions, eliminating the need for custom coding. This acceleration is critical for achieving time-to-value, with deployment often measured in a few months, rather than a year or longer, as is common with other alternatives.
- Legacy Cost: Since the vendor continuously maintains the system and enhances their products with new modules or AI capabilities, manufacturers are always running on the most recent, innovative version. This effectively eliminates the risk of technology obsolescence and the high cost associated with migrating old, unsupported platforms.
- Custom Performance: Customization is limited to configuration, APIs, and low-code extension tools provided within the platform’s framework. Deep code modification is not possible. However, some vendors do provide custom capabilities and dashboards for their clients. It is therefore recommended to inquire about such professional services if the standard SaaS solution does not cover specific digitalization needs.
Suitability: Which Pharma Manufacturer is the Best Fit for SaaS?
The SaaS model is optimized for rapid growth, multi-site standardization, and cost-effective digital transformation. Thus, it is highly suitable for:
- Small Manufacturers aiming to quickly increase throughput and market share without incurring high initial capital expenditure.
- Mid-sized and large manufacturers focused on rapid global scalability and achieving operational consistency across multiple sites.
- Manufacturers who recognize they cannot efficiently hire and retain world-class software engineers for designing, coding, and continuously improving the solution to stay competitive in the race for pharma AI.
- Generic and biosimilar producers where low-margin, high-volume production necessitates a cost-effective, digitally lean transformation.
2. Low-Code Platforms
Low-Code platforms enable users to build applications using visual, drag-and-drop interfaces and pre-built components, minimizing the need for traditional code development. In pharma manufacturing, these platforms are often used to quickly digitize manual workflows, create specific departmental tools, or bridge immediate data gaps between large, siloed enterprise systems.
Cost & Benefit Analysis
Low code tools offer significant speed and agility, but this comes with trade-offs regarding enterprise-grade performance and complexity.
- Labor & Maintenance Cost: The Labor Cost is lower than in-house development because it requires fewer specialized, high-wage developers. Application creation and maintenance are more focused on configuration, empowering citizen developers or business analysts. The vendor handles the core platform maintenance, reducing the overall IT operational burden. Nevertheless, costs tend to be higher compared to SaaS solutions.
- Scalability: Low code platforms offer very good scalability at the platform level. However, the applications built on the platform often face scaling challenges when subjected to the high-volume, real-time performance demands of core manufacturing processes. This makes low code applications unsuitable for enterprise-wide continuous improvement initiatives.
- Time & Ease of Deployment: Low code offers a fast time of deployment and ease of deployment especially if you have people with some coding skills. For simple, localized, non-core applications (like custom inspection forms), a functional solution can be prototyped and deployed in weeks. This agility provides excellent value for rapid iteration and meeting immediate departmental needs.
- Legacy Cost: This lands in a middle ground. While the vendor manages the core platform’s technology evolution, the primary Legacy Cost risk is vendor lock-in. If the pivots its strategy, or stops supporting the specific version, all applications built on that proprietary low code framework become instantly obsolete, leading to a costly re-platforming effort.
- Custom Performance: Is significantly restricted by the proprietary framework of the low code platforms. Deep process optimization or machine-level integration is challenging. A critical compliance challenge in pharma is System Validation: while the vendor validates the platform, every single application built on it must be individually validated by the manufacturer, which can dangerously multiply the validation workload and compliance risk across the organization. Another option is custom coding if not provided by the vendor negatively impacting time and ease of deployment as well as cost.
Suitability: Which Pharma Manufacturer is the Best Fit for Low-Code/No-Code?
Low code is best utilized as an agile, complementary toolset rather than the foundation for mission-critical Digital Factory systems.
Best For:
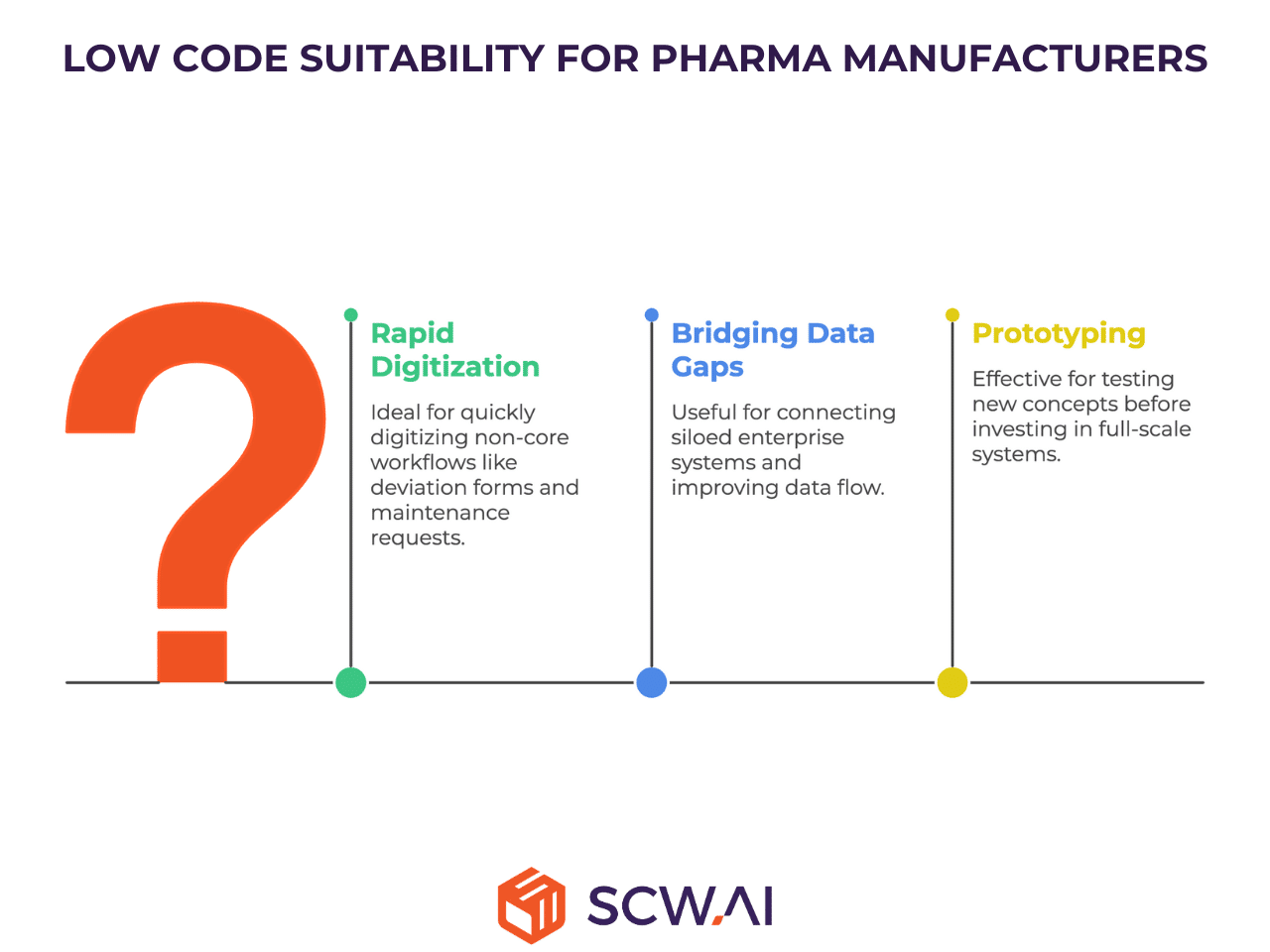
- Manufacturers of any size seek to rapidly digitize localized, non-core workflows (e.g., simple deviation forms, maintenance requests, or basic equipment tracking).
- Bridging data gaps between existing, complex, and often siloed enterprise systems.
- Prototyping and testing new concepts before committing to a full-scale, validated system investment.
3. On-Premise Solutions
The On-Premise model involves licensing the software (such as a SaaS platform) and deploying it on servers hosted within the manufacturer’s own data center or a dedicated private cloud environment. The key distinction is that the manufacturer retains full responsibility for managing the physical infrastructure, operating system, and often, the major maintenance and upgrade cycles. Therefore, on-premise systems carry hidden costs—much like an iceberg, the majority lie beneath the surface, and focusing solely on software licensing costs can be dramatically misleading.
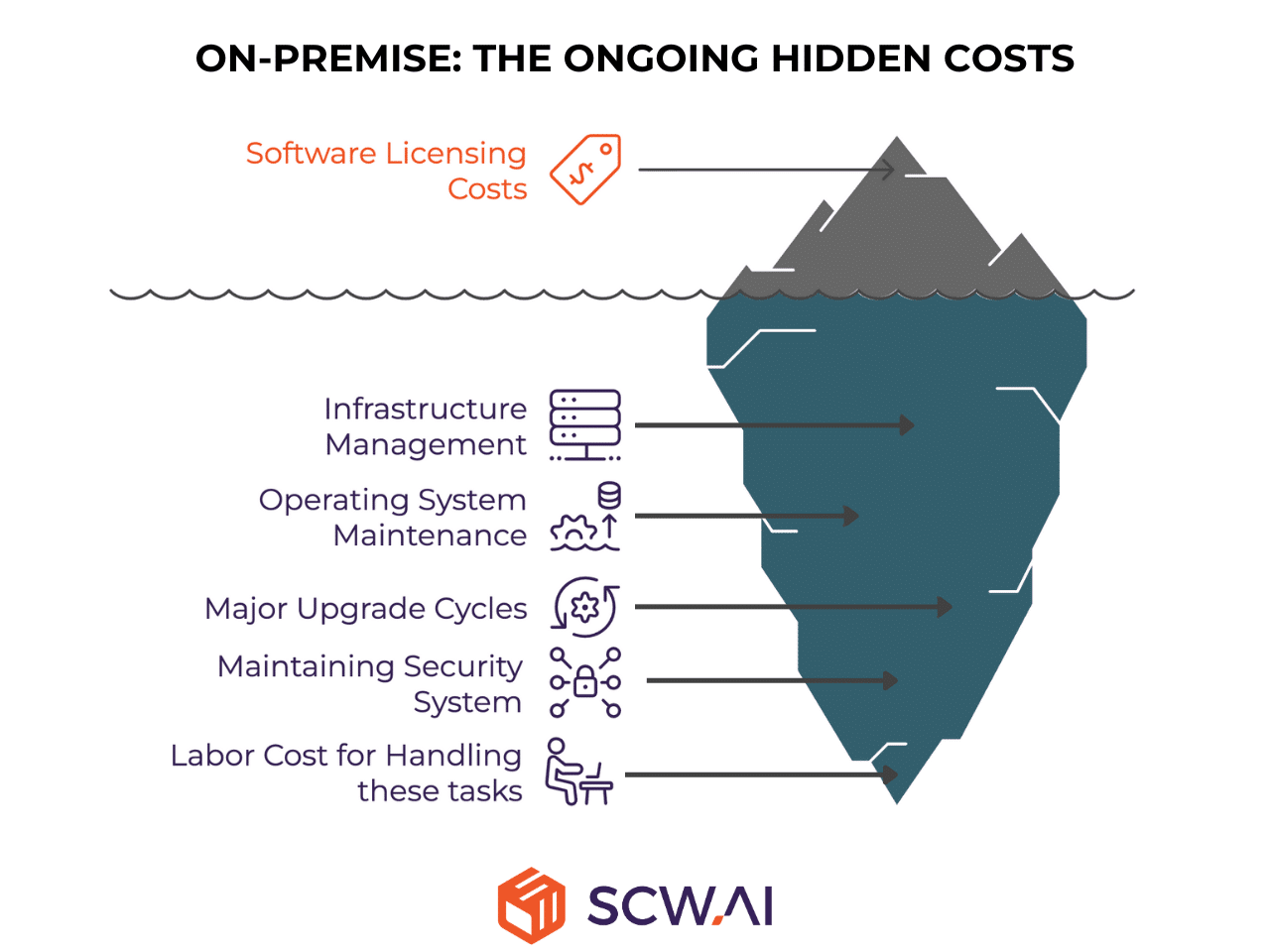
Cost & Benefit Analysis
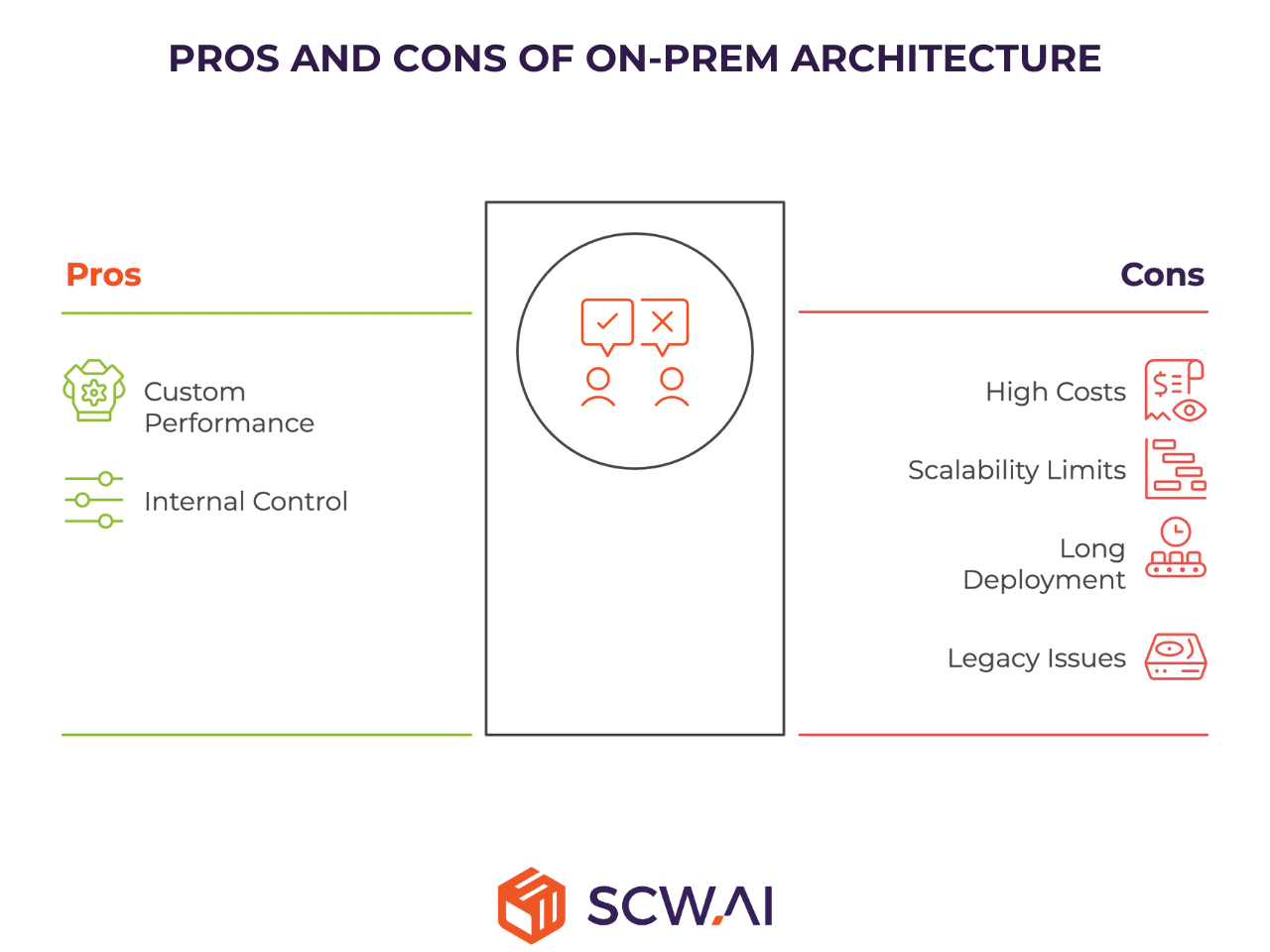
This model is characterized by high initial capital investment and high ongoing costs in exchange for internal control.
- Labor & Maintenance Cost: These costs are high. The manufacturer’s internal IT and Operations Technology (OT) teams bear the entire burden of Maintenance & Updates, including server patching, infrastructure management, and database administration. Dedicated personnel must be retained for the system’s operational continuity. Furthermore, every major software upgrade or update from the vendor necessitates a full, costly, internal System Validation cycle, significantly increasing labor effort.
- Scalability: Scalability is generally good but is restricted by the limits of the manufacturer’s physical infrastructure. Expanding capacity requires significant planning, capital expenditure on new hardware, procurement delays, and extensive internal implementation and validation efforts. This lack of elasticity makes sudden scaling challenging.
- Time & Ease of Deployment: Time of Deployment is medium to long. It is significantly slower than SaaS due to the lengthy internal process of hardware procurement, setting up the internal network, configuring the operating environment, and performing the initial comprehensive system validation and integration with existing OT/IT infrastructure.
- Legacy Cost: The Legacy Cost is high. While the manufacturer has control, they are responsible for ensuring the long-term viability of their chosen hardware and software versions. The cost and effort required to perform necessary major version upgrades often leads to delayed updates, increasing the risk of running on older, less secure, or eventually unsupported software.
- Custom Performance: On-Premise provides very good Custom Performance. Since the manufacturer fully controls the underlying infrastructure, they have flexibility to customize, integrate deeply with proprietary OT systems, and optimize performance parameters directly to suit highly specialized manufacturing processes. However, utilizing such capabilities require hire and retain high skilled IT professionals
Suitability: Which Pharma Manufacturer is the Best Fit for On-Premise?
The On-Premise model is a strategic fit for organizations whose core drivers are internal control, and leveraging massive existing infrastructure as well as IT professionals.
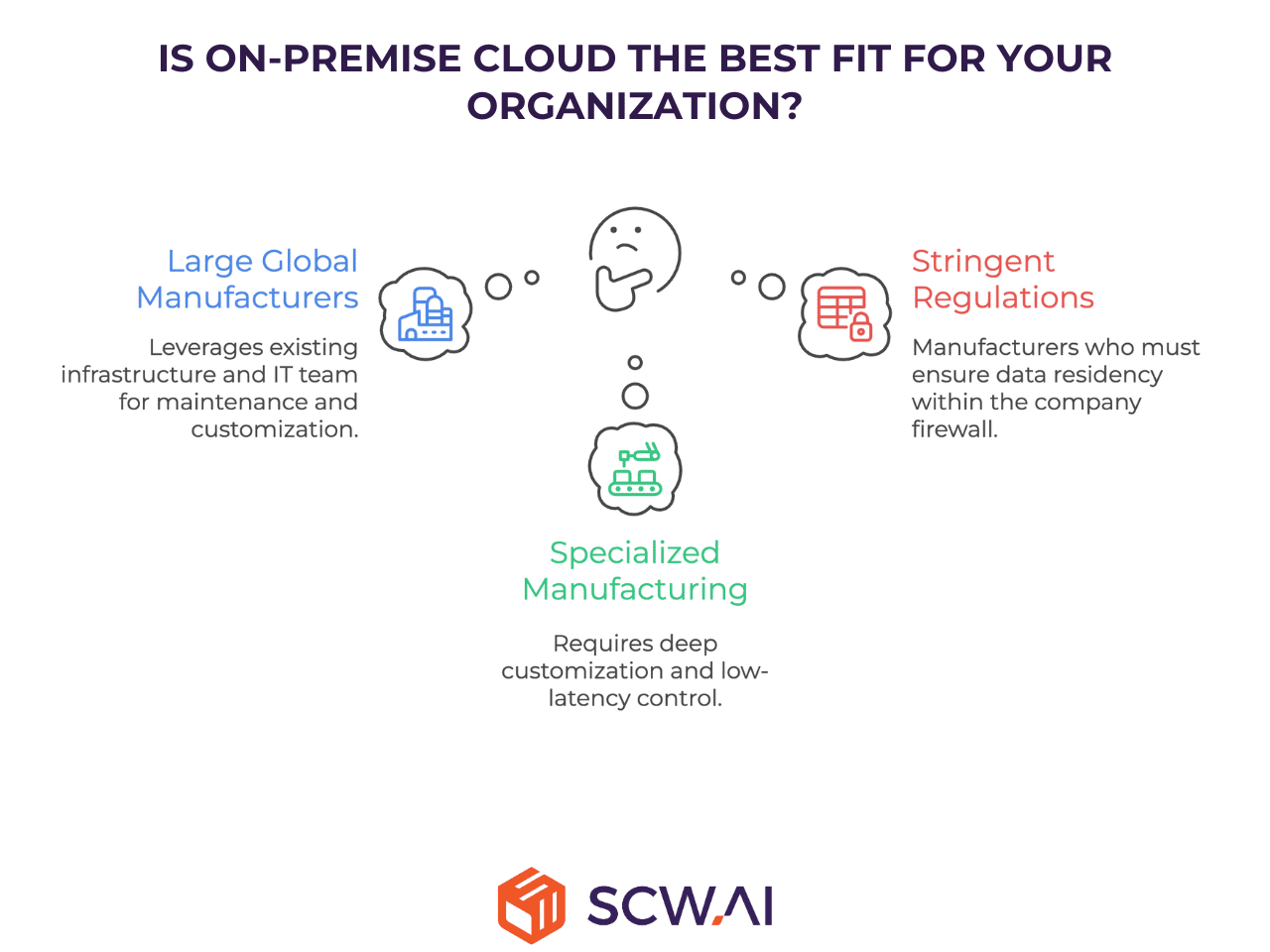
- Large, established global manufacturers with extensive, non-depreciated investments in internal server and data center infrastructure. This choice often assumes the long-term ability to retain a highly specialized internal IT team capable of supporting the demanding cycle of maintenance, custom code updates, and validation required for the on-premise solution.
- Operations with highly specialized or proprietary manufacturing processes that demand deep system-level customization and integration that no commercial cloud solution can support. This includes situations requiring direct, low-latency control of specialized manufacturing machines.
- Organizations subject to extremely stringent national or internal regulations that legally mandate data residency (data must never leave the company firewall).
A Note on Industry Trend
The trend across enterprise software, including core systems like Enterprise Resource Planning (ERP), which manages pharma supply chains, shows a clear shift away from on-premise solutions. For instance, major ERP vendors like SAP have signaled end-of-life for support on many of their traditional on-premise products (often extending only until around 2030), actively transitioning clients to their cloud offerings. If a manufacturer does not have a compelling, legal data residency requirement, switching to a cloud or SaaS model is now the strategic, future-proof approach to avoid being left behind.
4. In-House Solutions
In-House solutions are built from the ground up by the manufacturer’s internal development team or contracted developers, precisely tailored to the organization’s unique processes. This path represents the highest possible level of process customization but also cost and time to value.
Cost & Benefit Analysis
The In-House model offers unparalleled customization at a devastating long-term cost, primarily driven by the ongoing requirement for specialized, high-demand human capital.
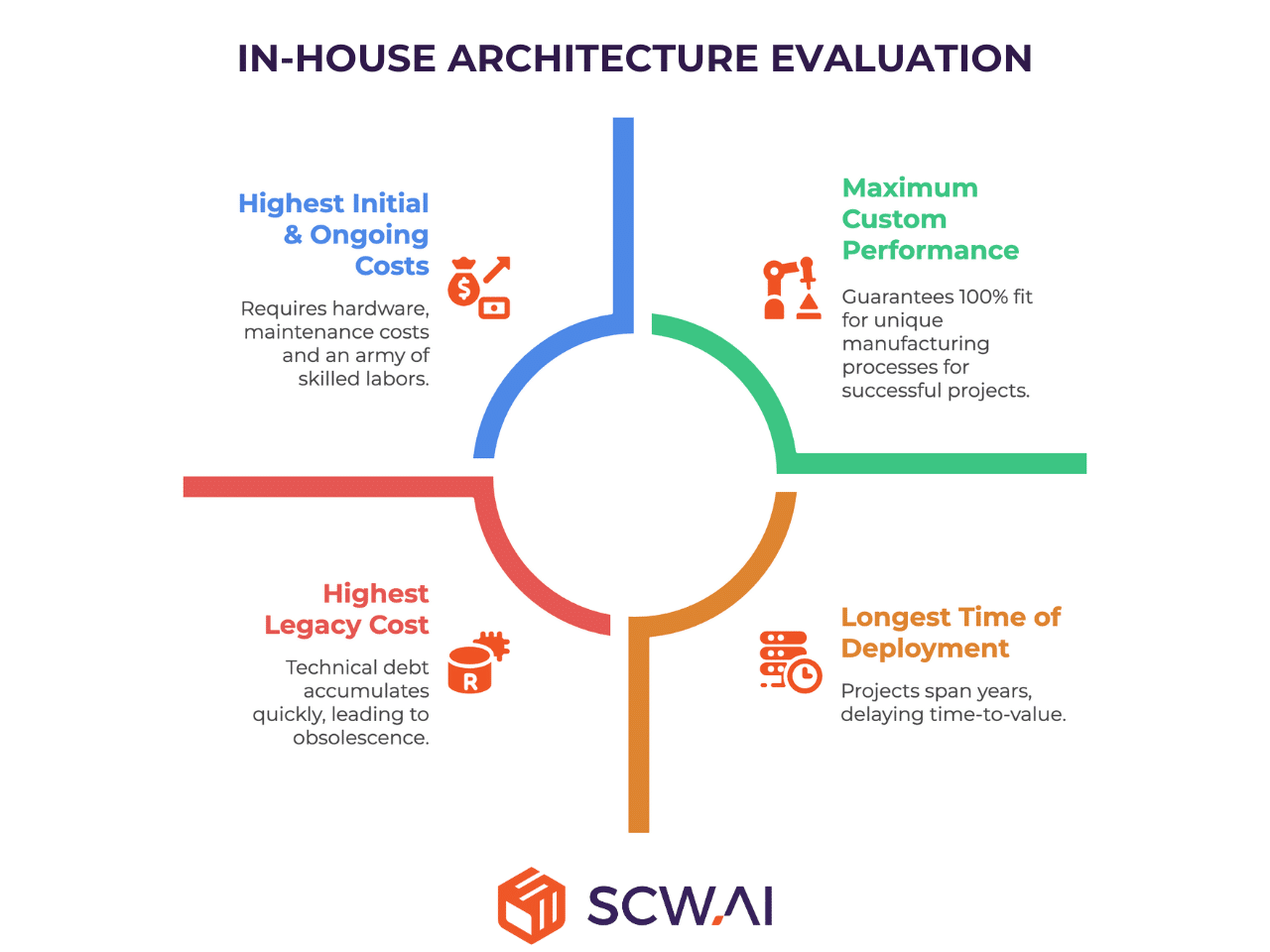
- Labor & Maintenance Cost: These costs are the highest. The internal team is solely responsible for every aspect of the software lifecycle, including architecture design, coding, testing, security, bug fixes, integration, and continuous System Validation. Crucially, this requires not just highly skilled coders, but also dedicated UX/UI designers and architects—today and in the future—to ensure the solution remains intuitive and efficient. Hiring and retaining this cutting-edge talent demands more than just salary; it requires a culture of innovation that many traditional manufacturing firms, including giants like BMW or Mercedes-Benz, struggle to provide completely, making employee retention a massive risk.
- Scalability: Is generally fair but expensive. Every expansion of capacity, feature set, or integration point requires bespoke re-engineering by the internal team. This manual, resource-intensive approach fundamentally lacks the elasticity and cost-efficiency of SaaS platforms.
- Time & Ease of Deployment: This model has the longest Time of Deployment and is the most complex. Projects often span multiple years, increasing financial risk and delaying time-to-value. The internal complexity of integrating new custom code with existing OT/IT infrastructure creates significant testing and validation hurdles.
- Legacy Cost: The Legacy Cost is the highest and most persistent risk. The internal team is forced to keep pace with the exponential growth in computing power and complexity (Moore’s Law). The cutting-edge solution built today becomes average in about five years and potentially obsolete in ten. The moment the internal development team slows its pace, or key personnel leave, the proprietary software immediately begins accumulating technical debt, often leading to a costly, unsupported platform that becomes a drag on innovation.
- Custom Performance: In-House delivers maximum Custom Performance. It is the only option that guarantees a 100% fit for highly proprietary, unique manufacturing processes that confer a distinct competitive advantage.
Suitability: Which Pharma Manufacturer is the Best Fit for In-House Solutions?
The In-House model should be approached with extreme caution and only when absolutely necessary under unique conditions.
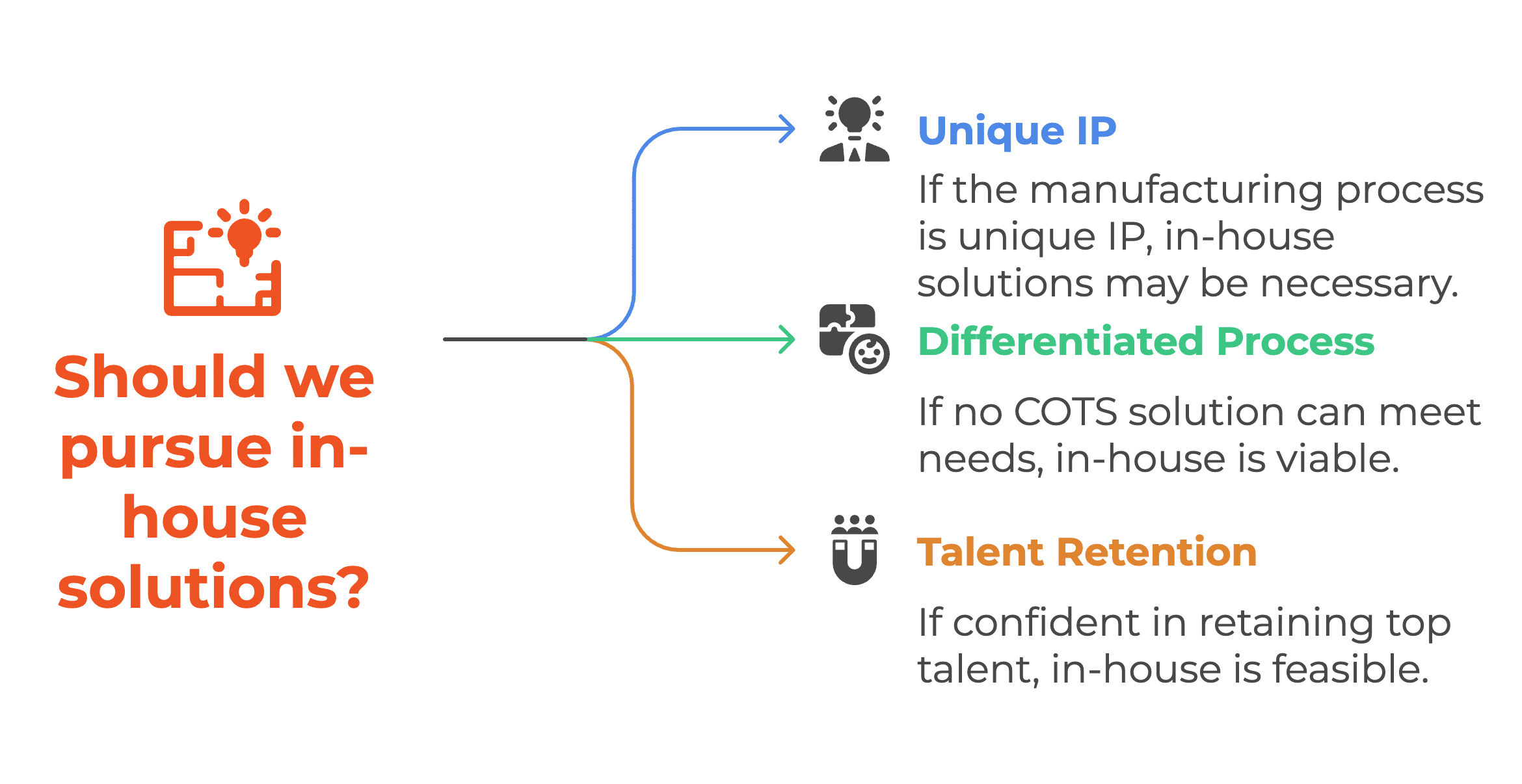
- Innovator drug companies whose core manufacturing process is itself a unique piece of intellectual property (IP).
- Situations where the process is so fundamentally differentiated that no Commercial Off-The-Shelf (COTS) solution, even with customization, can deliver the core operational functionality needed to maintain a competitive edge.
- Companies who are highly confident in their ability to hire and retain world-class software developers, AI developers, and UX/UI designers today and in the future. However, if the project depends heavily on a few key individuals rather than a sustainable corporate culture, the cost of losing this specialized personnel can risk the failure of the entire digital transformation journey.
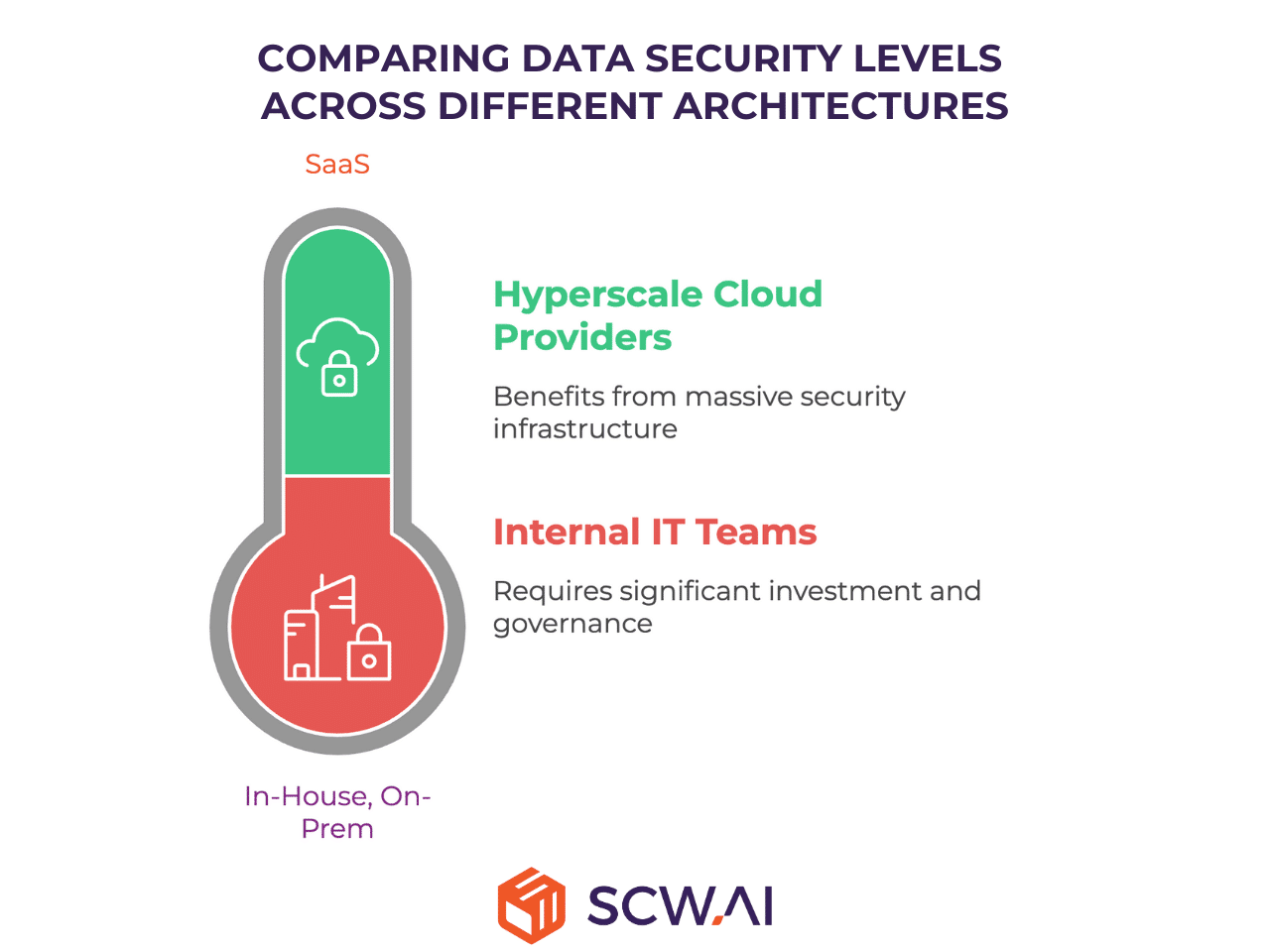
A Note on Data Security Criterion
In the comparison table, the Data Security criterion was rated highly and equally across all four architectures. This is a deliberate choice: today, robust security is non-negotiable and achievable by all vendors and internal teams.
However, it is crucial to recognize that for SaaS solutions leveraging hyperscale cloud providers (such as Microsoft Azure, AWS, or Google Cloud), the foundational level of security is often superior to that achievable by most manufacturers’ internal IT teams. These hyperscalers invest billions annually in cutting-edge security infrastructure and hold globally recognized compliance certifications. For instance, Azure handles data for the U.S. Department of Defense—an organization with arguably far greater data protection concerns than any typical pharma manufacturer. This reality means the underlying security of major SaaS platforms is frequently more robust than bespoke On-Premise or In-House systems.
Nevertheless, for the purpose of this strategic analysis, we maintain the equal rating, assuming that modern security standards are attainable across all four paths, provided the correct investment and internal governance are in place.
Conclusion and Next Steps for Your Digital Journey
The comparative analysis confirms that for the majority of pharma manufacturing organizations, the SaaS digital pharma manufacturing solution is the clear strategic winner. By externalizing the heavy burden of infrastructure, security, and maşntenace SaaS model provides cost leadership beyond agility.
If you’re interested in learning more about how our SaaS Digital Factory platform can provide end-to-end digital transformation for your manufacturing operations—covering monitoring, execution, compliance, planning, and sustainability—contact us today.
To see our platform in action and speak directly with our team, you can book a live demo.


