Compliance with Digital Logbook:
Pharma Paperless Quality Case Study
Key Results
Logbooks
Digitized
Digital Log
Entries Per Year
Reduction in Manual
Workload & Error Reduction
Our global pharmaceutical manufacturing partner, a U.S.-based company with billions in annual revenue. To strengthen GMP compliance and streamline operations, the company partnered with SCW.AI to digitize its paper-based logbooks. This digital transformation improves regulatory adherence while allowing workers to focus on more value-added tasks, reducing the time spent on manual data entry.
Scope
The project focused on replacing more than 2000 paper logbooks across solids and liquids manufacturing, as well as packaging operations, with a compliant paperless pharma manufacturing solution at one of the top 10 generic pharmaceutical manufacturing facilities.

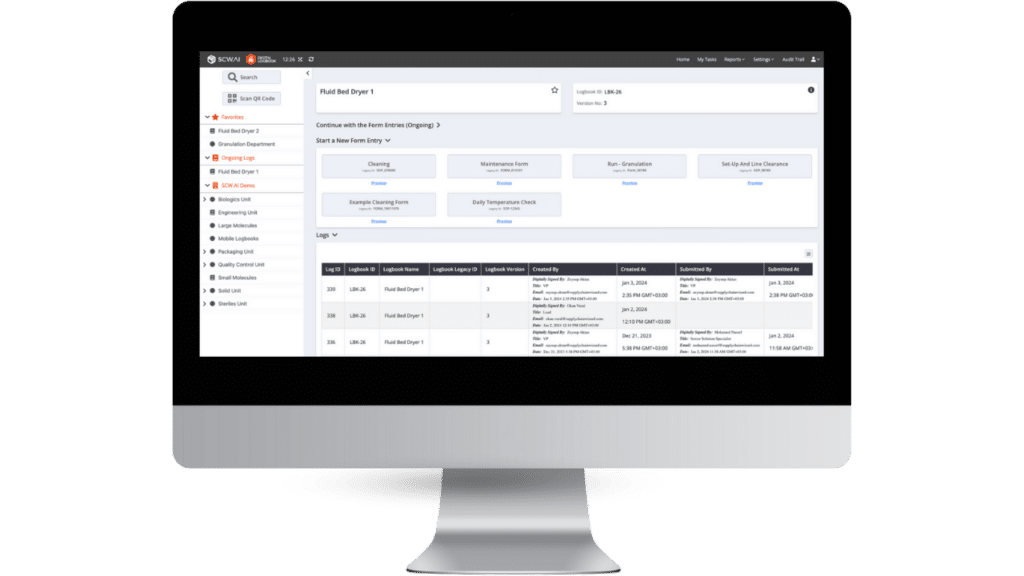
Challenges
- Heavy Reliance on Paper: Paper-based recording led to data loss and inaccuracies due to human error.
- Inability to consolidate data efficiently: Challenges in unifying, accessing, and managing data hindered reporting and decision-making processes.
- Negative impact on schedule adherence: Long cycles for data entry, review, approval, and reporting delayed operations.
- Resource drain due to manual compliance tasks: Highly manual work for regulatory and compliance processes resulted in significant resource losses.
Solution Implemented
To overcome its challenges, the pharmaceutical manufacturer implemented our GMP-compliant Digital Logbook, specifically designed to enhance compliance and efficiency in pharmaceutical operations. The Digital Logbook adheres to global regulations such as FDA 21 CFR Part 11, Eudralex volume 4 etc. for log entries, incorporating features such as e-signatures, built-in document management, a full audit trail, out-of-specification notifications, and more. Additionally, it improves data entry speed and accuracy with smart form features such as, automated calculations, and conditional workflows.

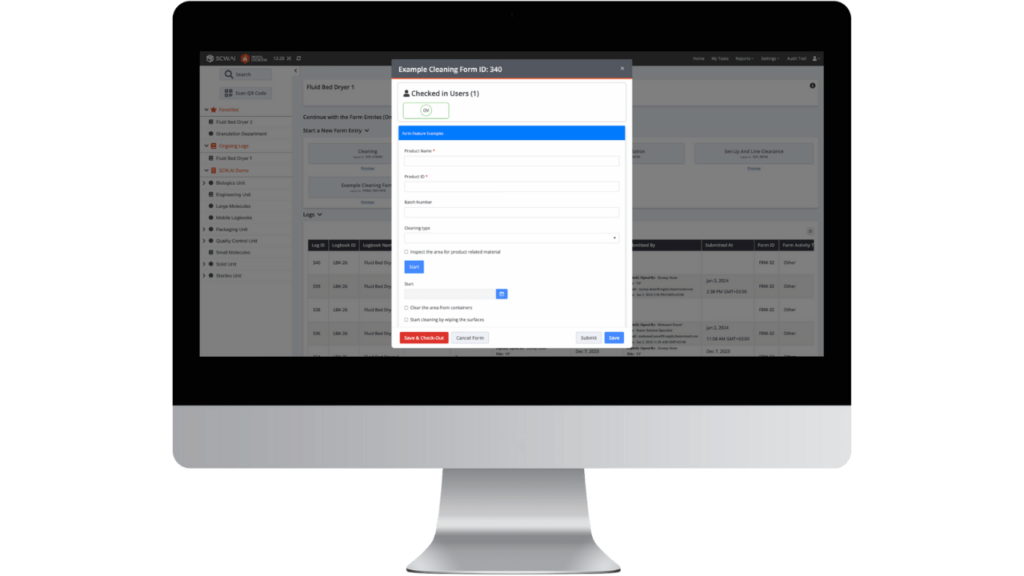
Operational Change
- Operators: Digital logbook simplified the data entry process, making it more straightforward and less stressful thanks to automated sections and predefined workflows.
- Production Executives: Logbook data became easily accessible. Digital factory transformation supported data-driven decisions and more effective root cause analysis.
- Quality Managers: Enhanced audit readiness thanks to more accurate data collection and secure, organized storage. Notifications for log entry approvals streamlined compliance processes. Creating new forms is quick and easy with drag-and-drop functionality, improving adaptability in response to new requirements.
Distinct Capabilities and Benefits
- Real-time data collection replacing paper logbooks.
- Improved audit readiness through automated compliance tracking.
- Reduced manual errors with automated workflows.
- Faster decision-making enabled by consolidated data reporting.
- Enhanced accountability through operator-driven error correction.
- Improved team engagement through real-time review processes.

Behind the Paperless Quality Transformation:
Working with SCW.AI
(USA)
(USA)
(USA)
(USA)
(USA)
(USA)
Download the PDF version of case study now. Use it as your inspiration for paperless quality transformation.
Ready to begin your own success story by transforming into a factory of the future? Discover how our Digital Factory Platform can revolutionize your operations. Book a demo today!
Sitemap
Resource Center
About SCW.AI
- ©2024 Supply Chain Wizard All Rights Reserved
- Privacy Policy
- Cookie Policy
- Terms of Service