Paperless manufacturing offers significant business advantages, including cost reduction, decreased data entry time and errors, support for sustainable practices and more. However, pharmaceutical manufacturers must adhere to stringent regulations such as FDA 21 CFR Part 11, necessitating GMP compliance and the assurance of ALCOA+ data integrity principles in log documentation.
Acknowledging the distinct regulatory landscape of the pharmaceutical industry, SCW.AI has developed the GMP compliant Digital Logbook—an industry-tailored paperless factory solution. This article will first introduce the GMP compliant Logbook, outlining its key capabilities that position it as a valuable digitalization asset for pharmaceutical companies. Following that, we will spotlight the top 5 use cases of the tool to underscore its business benefits, including how our Digital Logbook is capable of reducing the likelihood of receiving a warning letter from the FDA by around 6%.
An Introduction to GMP Compliant Digital Logbook
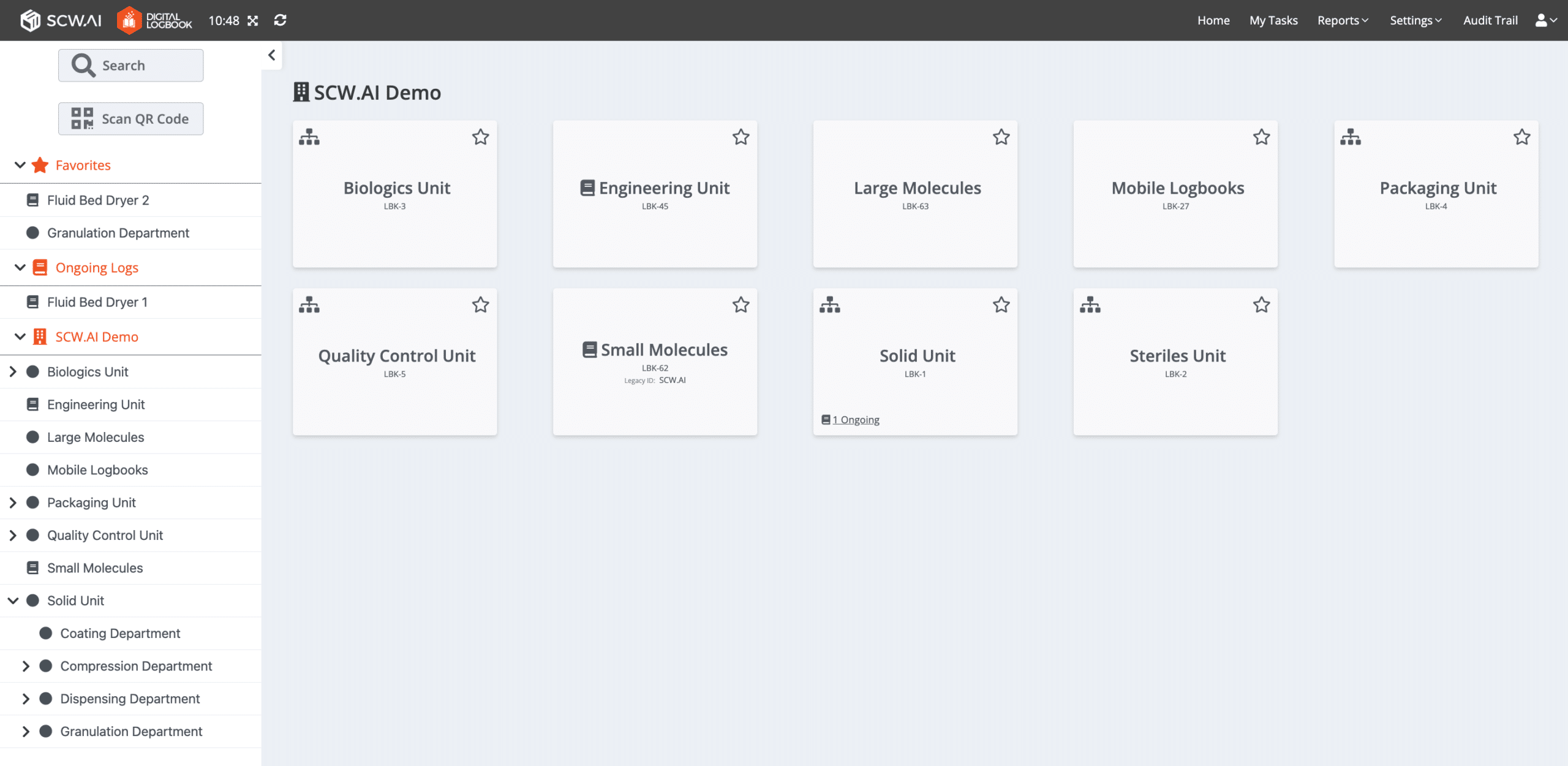
The Digital Logbook or Electronic Logbook, is SCW.AI’s cloud-based tool designed to assist manufacturers in managing, coordinating, reporting, and auditing production logs more effectively compared to the paper based documentation.
Our Digital Logbook is specifically tailored for the pharmaceutical industry, adhering to the industry’s regulations governing documentation practices. In this context, it offers enhancements in terms of data integrity and versioning capabilities compared to standard paperless manufacturing software.
The most significant distinction lies in SCW.AI’s digital logbook being a GMP-compliant solution that adheres to ALCOA+ data integrity principles, ensuring:
- Each data record in the solution is attributable to a specific role that can be defined as desired (e.g., operator, line leader, supervisor, production manager).
- Data is stored in a way such that it is always easily retrievable, understood and audit-ready.
- Archiving ensures that all past entries are securely stored and easily accessible, which is important for data analysis and legal compliance.
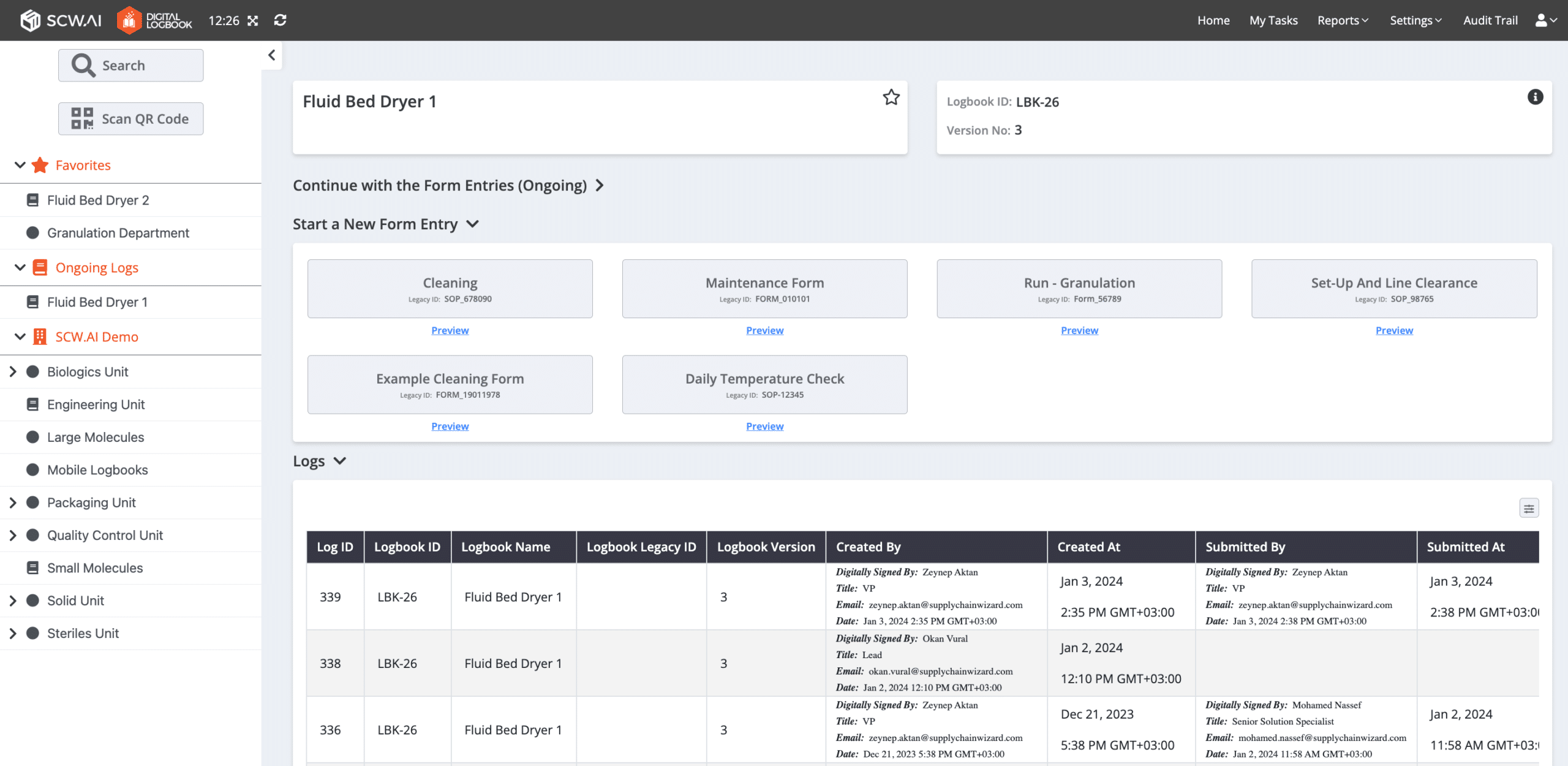
10+ Features of Digital Logbook
To showcase the full capabilities of the Digital Logbook, let’s delve into its main features below.
GMP Compliant Features
The initial 8 features of the Digital Logbook are meticulously designed to comply with GMP:
1. User Authentication
Each action taken in the system prompts the user to provide user-specific credentials.
2. E-signatures
Each user possesses a unique e-signature, offering a secure and traceable method for signing off on entries.
3. Access Management
Tailors access rights for various roles within the organization, ensuring data confidentiality segregation of duties.
4. Built in Document Management System
Centralizes document storage and retrieval within the Digital Logbook, promoting organization and adherence to chronological accuracy for data entries.
5. Full Audit Trail
Facilitates easy access to critical documents for audits and inspections. Auditors from FDA, EMA etc. can efficiently check specific log entries for a particular date and work order with just a few clicks.
6. Out of Specification Notification
Proactively identifies and notifies deviations in real-time, facilitating swift corrective action and maintaining product quality standards.
7. Data Completeness
Ensures the completeness of log entries by highlighting mandatory fields, promoting comprehensive and accurate data recording.
8. Captures Log Start-End Times
Accurately records and displays timestamps for each log entry, supporting chronological tracking and compliance with data integrity principles.
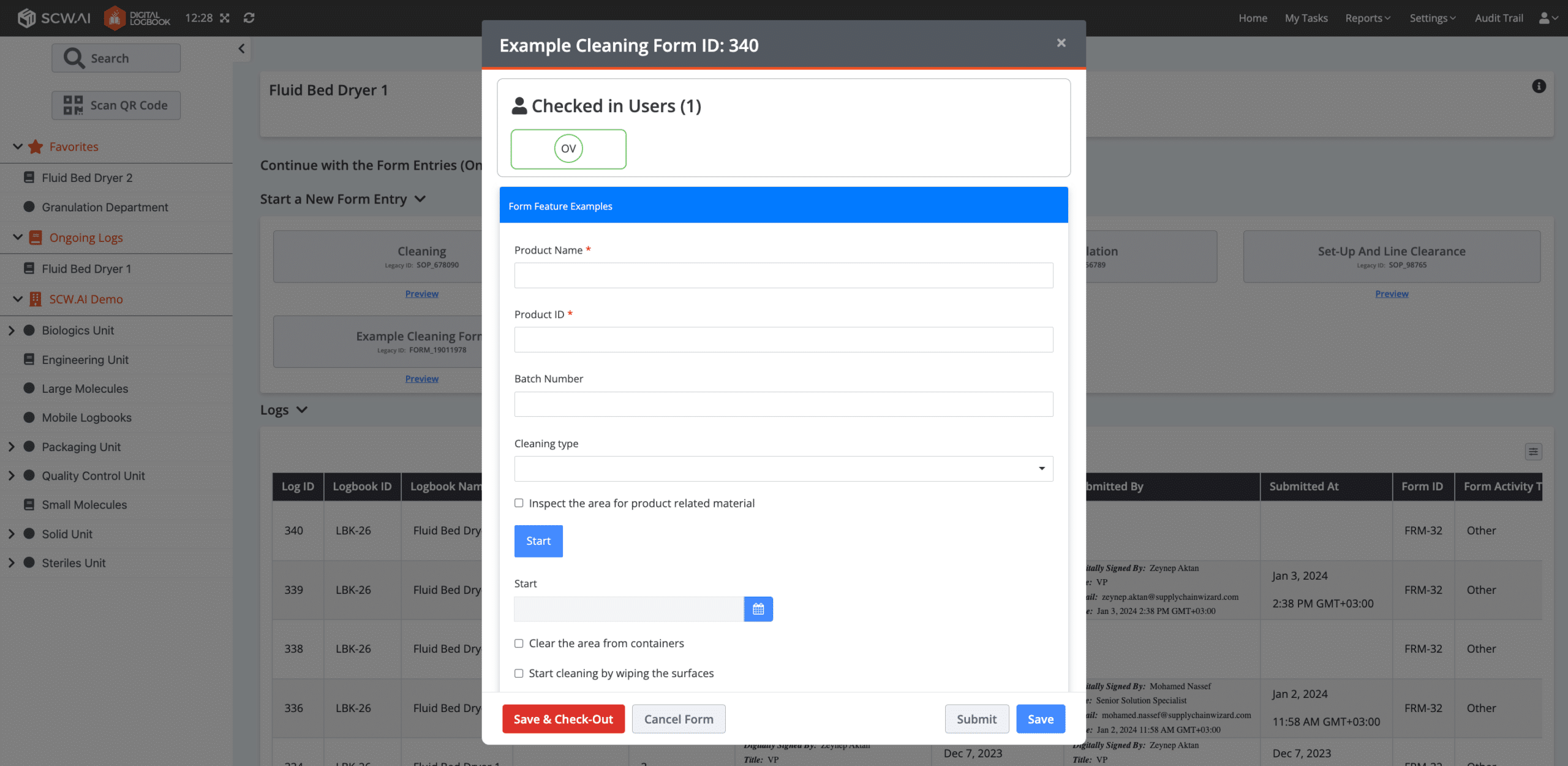
9. Automated Calculations
A smart form capability that assists users by providing automated calculations based on user input. In this sense, the likelihood of incorrect data entry pertaining to computed areas becomes null, improving the quality and accuracy of the data.
10. Conditional Workflows
By automating specific data entry tasks based on predefined conditions or criteria, such as production line activities or outputs, conditional workflows eliminate the need for manual intervention, ensuring accurate and timely documentation.
11. Automated Data Capturing
SCW.AI’s Digital Logbook offers seamless integration with IoT devices featuring PLC connections. This integration provides manufacturers with enhanced visibility into the shop floor, offering real-time insights into labor check-ins, machine statuses, electrical currents, and environmental conditions such as temperature and humidity.
IoT integration empowers pharmaceutical manufacturers to automate some portion of data entry processes. For example, in scenarios where recording the temperature of a production line is critical, the GMP compliant logbook facilitates automatic data entry for this specific parameter.
Furthermore, Digital Logbook is designed to effortlessly integrate with existing ERP, MES, and LIMS tools, eliminating any additional legacy costs. Instead, it serves as a valuable bridge for your documentation activities, enabling automatic data capture from your pre-existing digital infrastructure.
12. Real-Time Notifications
Digital Logbook is equipped with real-time notification capabilities, ensuring that users receive instant alerts of tasks to be completed. This feature serves executives by keeping them informed about potential bottlenecks on the shop floor, allowing for prompt decision-making and quick approvals. Managers can effortlessly track tasks that require their attention, facilitating efficient task completion and approval within the realm of quality management.
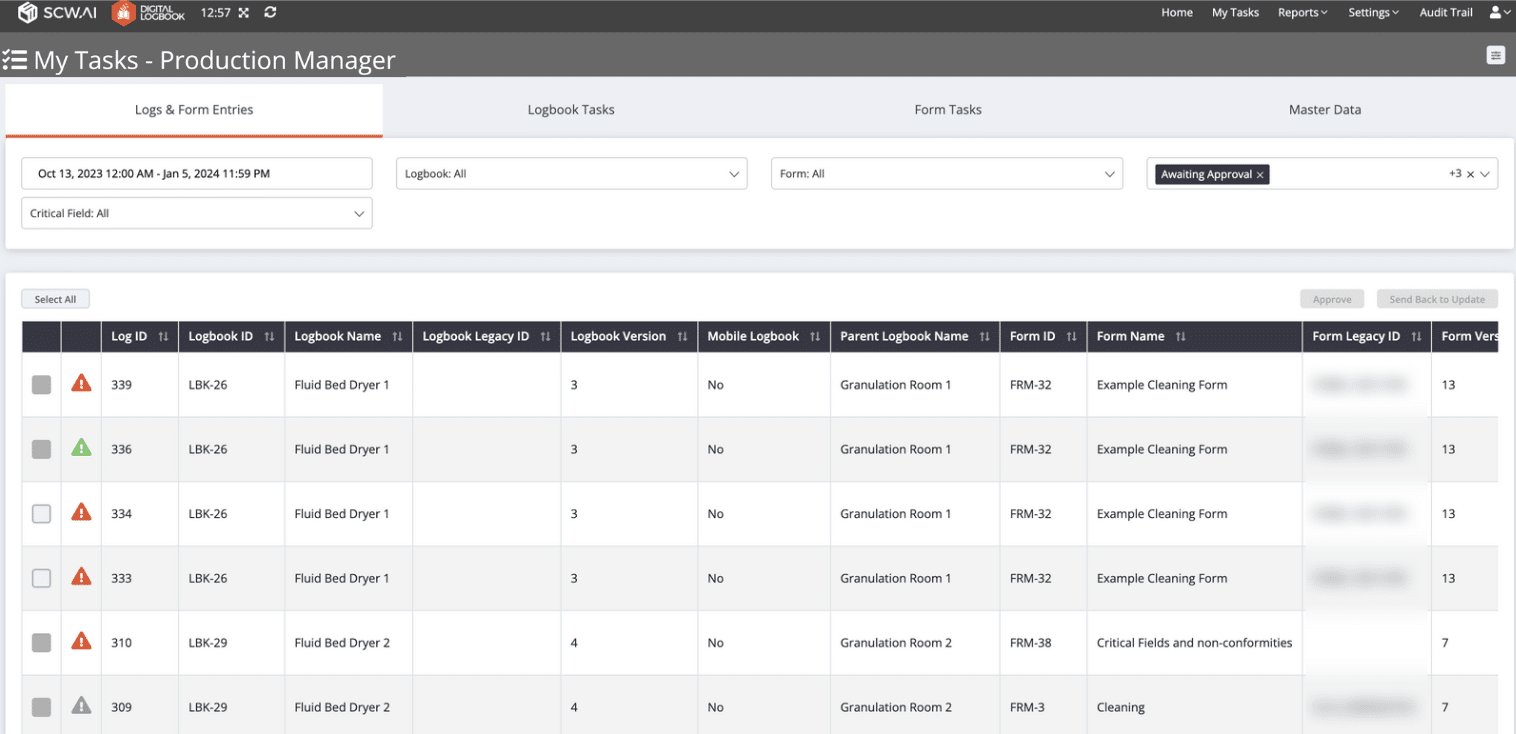
13. Drag and Drop Form Building
Digital Logbook offers administrators a user-friendly experience in crafting forms tailored to their specific workflows. The intuitive drag-and-drop functionality simplifies the process of building forms.
14. Link to References and Upload Attachments
The Digital Logbook system offers support for documentation needs by enabling users to link to references such as SOPs and documents to forms. Additionally, it facilitates the upload of files and images during log entries, allowing for detailed illustration and explanation of production activities, such as demonstrating completed line cleaning and highlighting bottlenecks to supervisors and managers.
Top 5 Use Cases of Digital Logbook

1. Reduce the Risk of Receiving Warning Letters by 6%
In 2023, the FDA issued 1,383 warning letters. Among these, 83 warning letters, or 6% of them, were related to non-compliance with ALCOA+ data integrity principles. These issues included:
- Unclear user authentication
- Ineffective or non-existent built-in document management systems with version controls
- Lack of access management or e-signatures
- Failure to fill mandatory fields
- Absence of a mechanism for out of specification notification and more.
From this perspective, the Digital Logbook is an investment that enhances corporate compliance, reducing the likelihood of receiving warning letters from the FDA by approximately 6%. This level of security is crucial for pharmaceutical companies, as receiving warning letters from the FDA or similar institutions is a harbinger of bad news. These regulatory bodies carefully review corrective action plans and assess whether adequate measures are implemented within specified time frames. Non-compliance with regulations may result in penalties and product recalls, causing financial and reputational damage to companies.
FDA issued 1383 warning letters in 2023
83 warning letters or 6% of them could have been avoided by Digital Logbook
2. Minimize Costs
Paper incurs both direct and indirect costs for manufacturers. Activities such as purchasing, printing, and storing paper contribute to expenses. However, the most substantial cost associated with traditional paper-based documentation lies in indirect expenses.
According to McKinsey, documentation-related activities for pharmaceutical companies can consume up to 30% of operator time. Prolonged documentation processes lead to operators spending more time on non-value-adding tasks, ultimately impacting their motivation. There are frequent anecdotes, expressed in various languages, where operators humorously refer to GMP as something akin to the “Great Mass of Paper.”
Contrastingly, based on our experience, the utilization of the Digital Logbook can reduce documentation time by approximately 50% to 85%, depending on the task. This reduction in time spent on documentation contributes to a 30% to 40% increase in the time dedicated to value-adding tasks by operators.
3. Produce Higher Quality Products
The Digital Logbook facilitates a comprehensive analysis of shop floor tasks, allowing managers to scrutinize deviations during specific time periods. This enhanced visibility empowers management to identify associations between production quality and various factors such as specific production lines, machines, shifts, and individual workers.
By leveraging the detailed insights provided by the Digital Logbook, managers can strategically address quality-related issues. For instance, if a particular production line consistently exhibits deviations, proactive measures can be taken to maintain and optimize the associated machinery. Similarly, identifying patterns across shifts or specific workers allows for targeted training programs to improve performance and adherence to GMP standards.
4. Eliminate the Environmental Cost of Paper
According to a Science Direct article, the pulp and paper industry stands out as one of the most environmentally detrimental sectors, characterized by excessive water consumption, high CO2 emissions, and detrimental effects on ecosystems through deforestation. A forecast for 2024 predicts the destruction of over 110,000 square kilometers of forests for paper production, an area nearly equivalent to the size of Pennsylvania or one-third of the entire Germany.
In an age where paperless solutions, such as SCW.AI’s Digital Logbook, are readily available, and stakeholders including workers, investors, consumers, and regulatory bodies actively seek environmentally, socially, and governance (ESG) initiatives from companies, participating in the pollution caused by the paper industry becomes unnecessary.
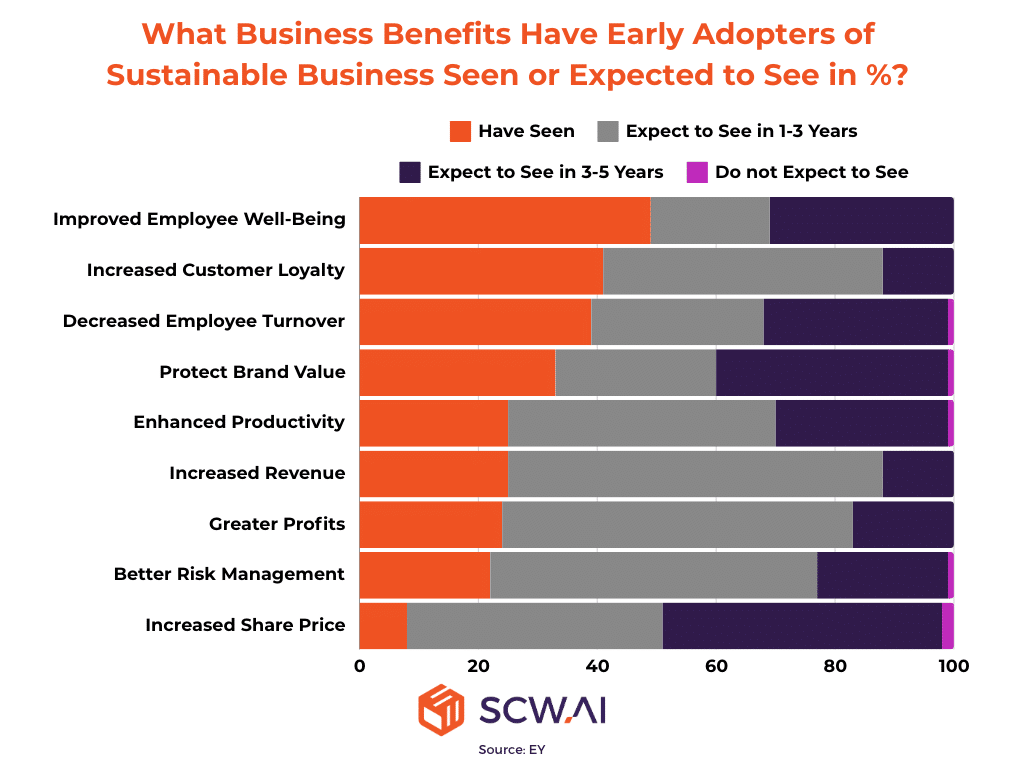
The accompanying image from a recent EY study illustrates the tangible business benefits experienced or anticipated by early adopters of sustainable business practices. It illustrates that ESG practices constitute profitable investment areas for businesses, enhancing the likelihood of attracting and retaining high-quality workers, improving productivity, fostering customer loyalty, and positively influencing share prices, among other advantages.
5. Hire and Retain Fresh, Talented Workers for Your Organization
According to the National Association of Manufacturers, the most significant challenge for manufacturers in 2023 was hiring and retaining talented workers. This issue is closely linked to the manufacturing industry’s struggle to attract Gen Z and Millennials to their workforce. Additionally, as the baby boomers retire due to their age, this contributes to labor shortage problems for manufacturers, including those in the pharmaceutical industry.
A Forbes article underscores that embracing paperless manufacturing could serve as a solution for manufacturers seeking to attract younger generations. Given that Gen Z and Millennials are digital natives, having grown up in a digital era, they naturally gravitate towards digital tools in various aspects of their lives, from everyday shopping to banking activities. Consequently, incorporating digital processes into professional workflows becomes an appealing proposition for these generations and generates an unexpected use case for GMP compliant Digital Logbook.
Paperless Pharmaceutical Manufacturing with SCW.AI
In the realm of pharmaceutical manufacturing, SCW.AI offers specialized solutions, including the Digital Logbook and Digital Batch Records, aimed at ensuring a streamlined process for document creation, management, and storage. These paperless quality solutions seamlessly integrate with the visibility, productivity, and agility solutions provided by SCW.AI’s Digital Factory, such as OEE Tracker, Labor Tracker, AI Scheduler, and more. This integration offers pharmaceutical manufacturers a comprehensive solution package.
To delve deeper into the capabilities of our Digital Logbook and the Digital Factory Platform, we invite you to book a demo and discover how these solutions can revolutionize your pharmaceutical manufacturing processes.
To learn further regarding how digital factory transformation can improve your compliance, flexibility and productivity you can contact us.