The global pharmaceutical landscape is undergoing a profound transformation, and at its heart, India’s Contract Development and Manufacturing Organization (CDMO) market is emerging as a pivotal force. For top managers and factory leaders in Indian pharma, understanding this shift is not just about staying competitive; it is about seizing a generational opportunity to redefine India’s role as the “Pharmacy of the World.”

This blog post offers a strategic overview, drawing insights from our comprehensive, free white paper, “India CDMO Market White Paper: A Roadmap to Becoming a Leader in Pharma Manufacturing.” We will explore the dynamic growth drivers, acknowledge the critical challenges, and highlight how Pharma 4.0 technologies in India are providing a robust roadmap for future readiness and global leadership.
India's Unstoppable Rise in Global Pharma
The narrative of Indian pharmaceutical manufacturing in the 21st century is one of remarkable acceleration. The Indian CDMO market has not merely grown; it has surged, doubling its market size between 2019 and 2023 to reach an estimated $20 billion, demonstrating an impressive 18% Compound Annual Growth Rate (CAGR). This expansion underscores the sector’s crucial contribution to India’s broader pharmaceutical industry, which already supplies a significant portion of the world’s generic drugs and vaccines. This growth trajectory is integral to India’s ambitious India@100 vision, which targets a $450 billion pharmaceutical market revenue by 2047.
Several powerful forces are propelling this ascent:
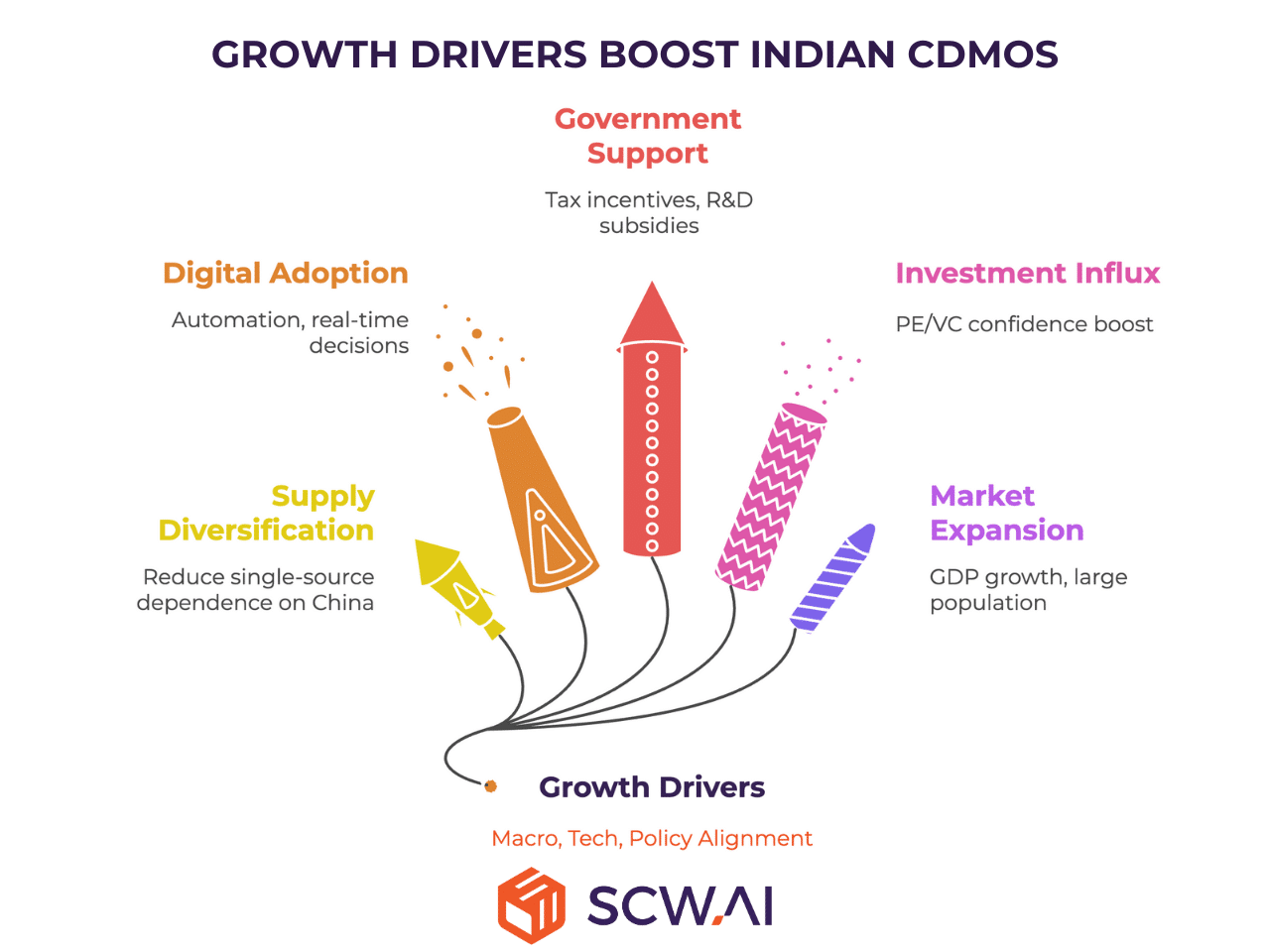
Strategic Supply Chain Diversification
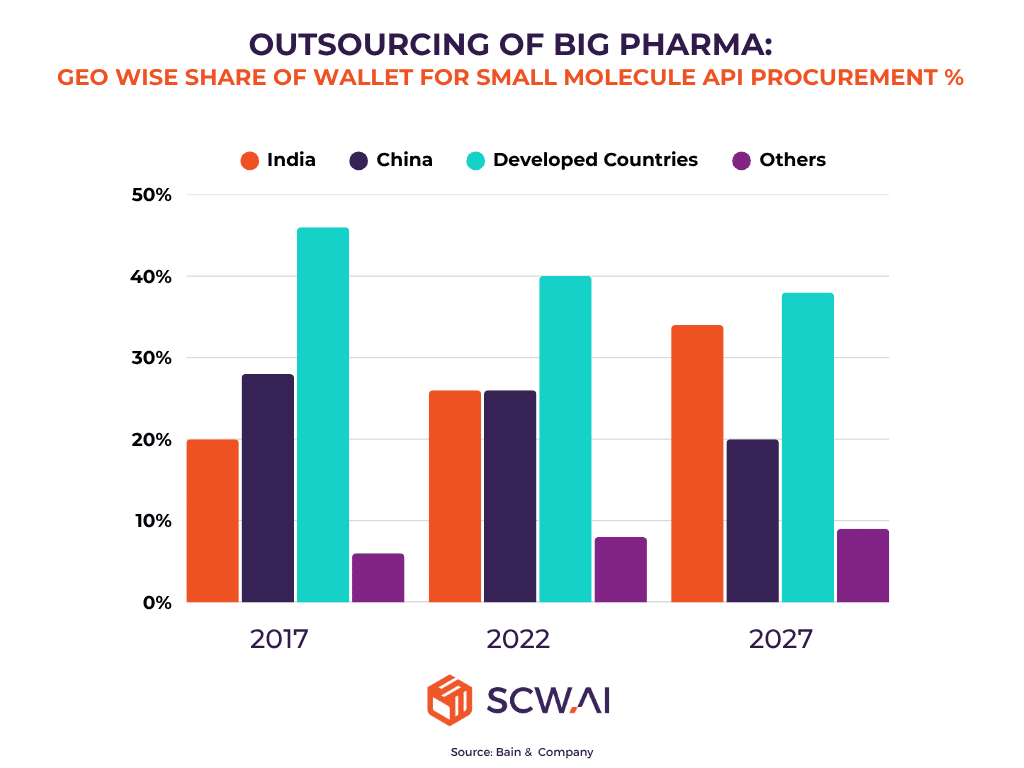
Global events, including geopolitical realignments and the lessons learned from recent supply chain disruptions, are compelling major pharmaceutical companies worldwide to reduce their reliance on single-source suppliers. Initiatives like the U.S. Biosecure Act and the EU’s Clean Industrial Deal are actively encouraging the diversification of pharmaceutical supply chains, particularly away from China.
India, with its mature manufacturing ecosystem, cost-competitive production, and strong international relations, is uniquely positioned to benefit from this strategic pivot. This shift is not merely theoretical; leading analyses project a substantial increase in India’s share of global outsourcing, indicating a clear strategic preference for Indian partners in the coming years. This represents a monumental opportunity for Indian CDMOs to capture a larger portion of the global market.
Leveraging India's Digital Acumen and Pharma 4.
With a large, young, and digitally fluent population, India possesses an inherent advantage in adopting advanced technologies. While the pharma industry globally has been slower to digitize, India’s proactive embrace of Pharma 4.0 technologies is setting new benchmarks.
Proof of this leadership is evident in the recognition of several Indian pharmaceutical factories as “Lighthouses” by the World Economic Forum. These facilities are global pioneers in applying Fourth Industrial Revolution technologies at scale, demonstrating how digital integration can lead to significant improvements in efficiency, quality, and overall output. This digital readiness enables Indian CDMOs to implement real-time insights, intelligent automation, and advanced analytics, providing a formidable competitive edge.
Government Support and Robust Investment
The Indian government has unequivocally recognized the pharmaceutical industry as a strategic pillar within its ambitious India@100 vision. This commitment translates into tangible support through various incentives, including tax deductions for R&D, subsidies for infrastructure, and the impactful Production Linked Incentive (PLI) schemes. These policies are increasingly tailored to directly benefit CDMOs in India, fostering an environment conducive to growth and self-reliance.
Complementing government initiatives, private capital is also flowing robustly into India’s healthcare sector. Private Equity and Venture Capital (PE/VC) investments in healthcare have surged, making it one of the fastest-growing investment domains in the country. This strong investor confidence underscores the long-term potential of India’s pharmaceutical ecosystem and provides crucial capital for Indian CDMOs to scale operations, modernize infrastructure, and invest in advanced technologies.
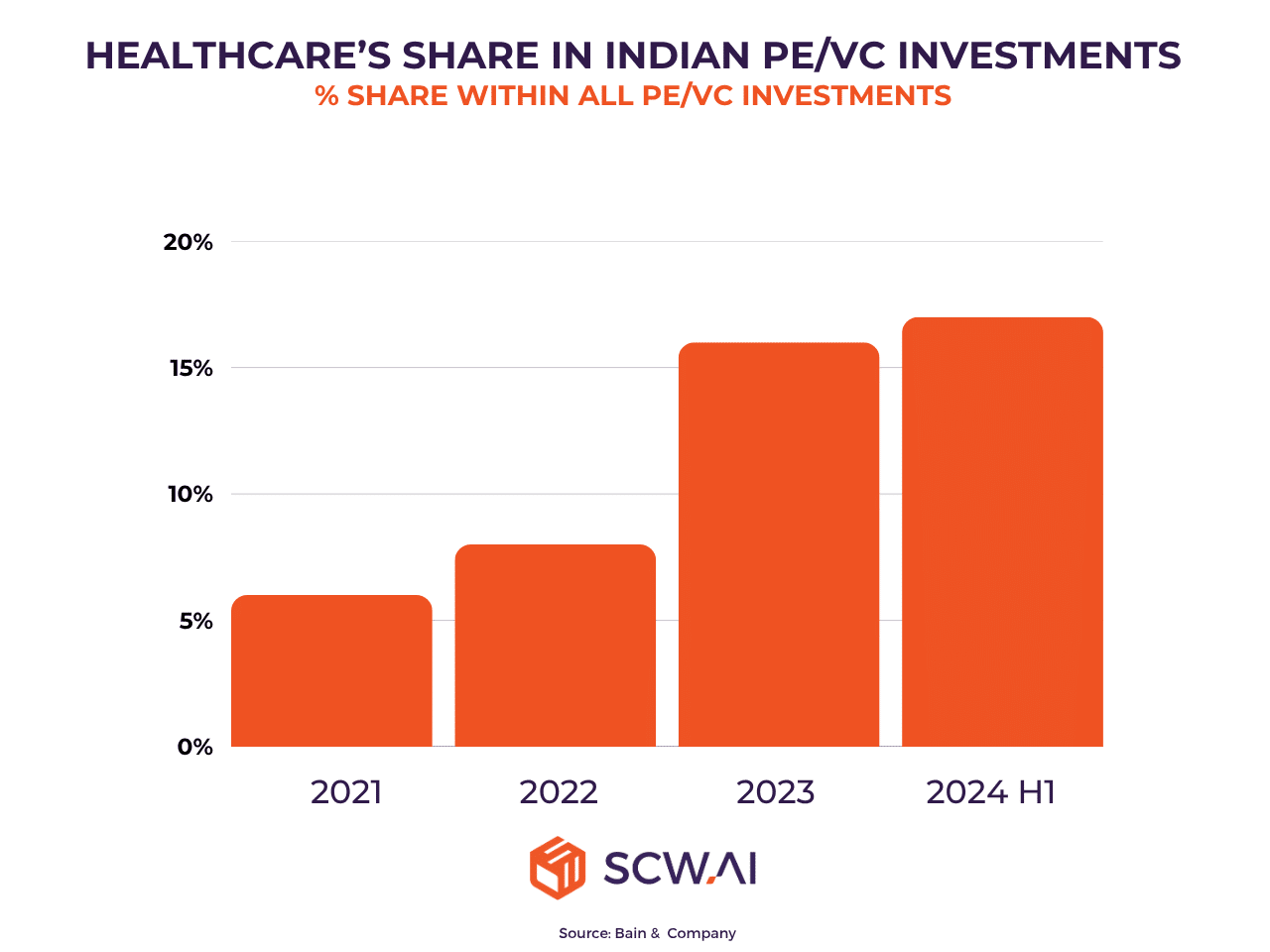
Expanding Domestic Market Potential
Beyond exports, India’s domestic pharmaceutical market is poised to become a significant growth engine in its own right. With the world’s largest population and a rapidly increasing GDP per capita, the correlation between economic development and pharmaceutical consumption is undeniable. As India transitions towards an upper-middle-income economy, a burgeoning middle class will drive exponential demand for a wider range of therapies. This growing internal market provides a resilient foundation for Indian CDMOs, complementing their export ambitions and ensuring sustained demand.
Navigating the Road Ahead: Key Challenges
While the growth prospects for Indian CDMOs are compelling, the path to global leadership is not without its complexities. Our white paper delves into critical challenges that require strategic foresight and proactive mitigation.
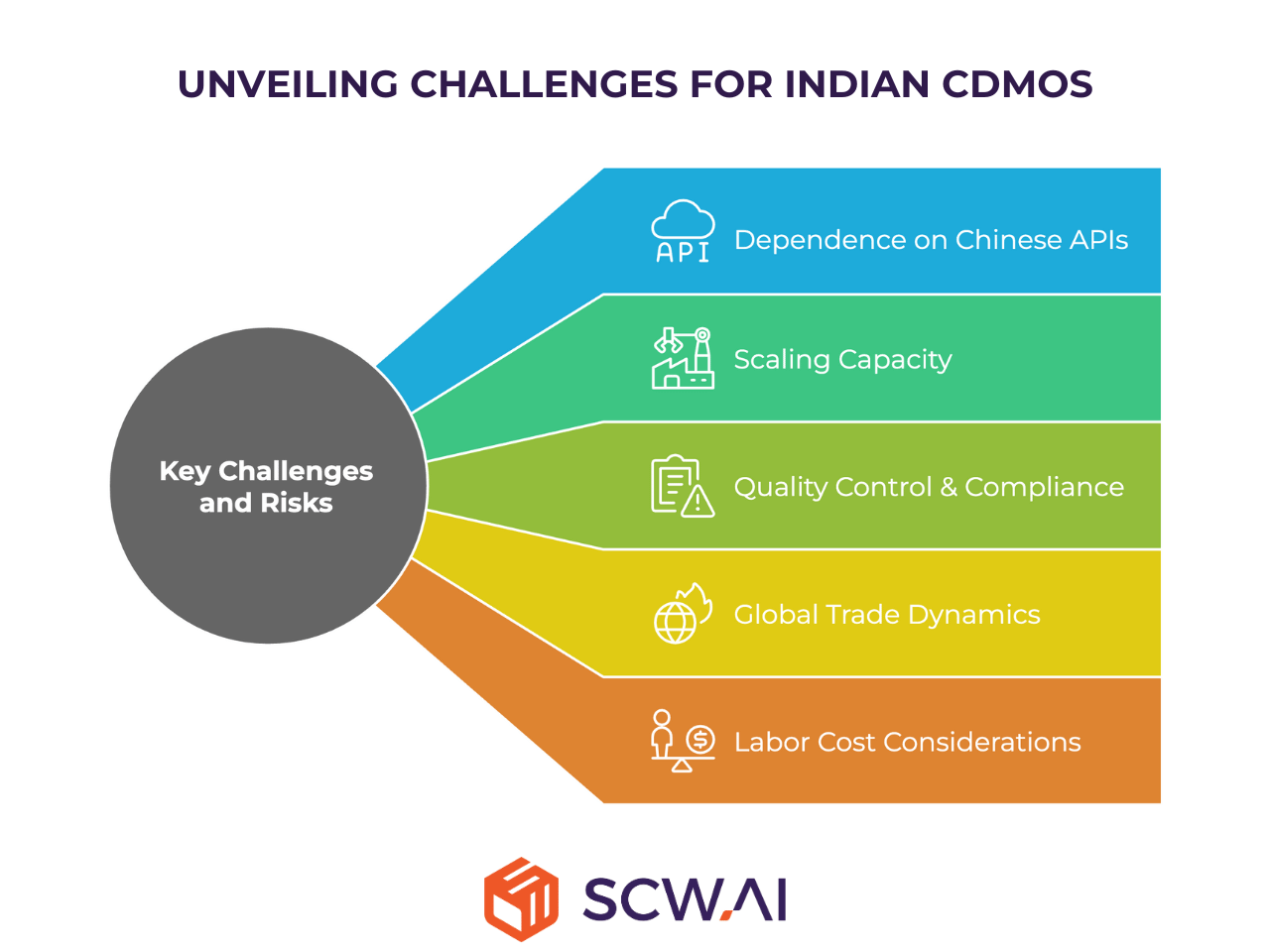
Dependence on Chinese APIs
A significant short-term vulnerability for Indian CDMOs is their continued reliance on Chinese imports for Active Pharmaceutical Ingredients (APIs). Approximately 75% of India’s API needs are currently met by China. This dependency creates strategic exposure, geopolitical alignment concerns, and risks related to trade friction and tariffs, which could impact the stability and competitiveness of Indian pharmaceutical manufacturing. Addressing this requires a dual strategy of investing in domestic API production and diversifying sourcing geographically.
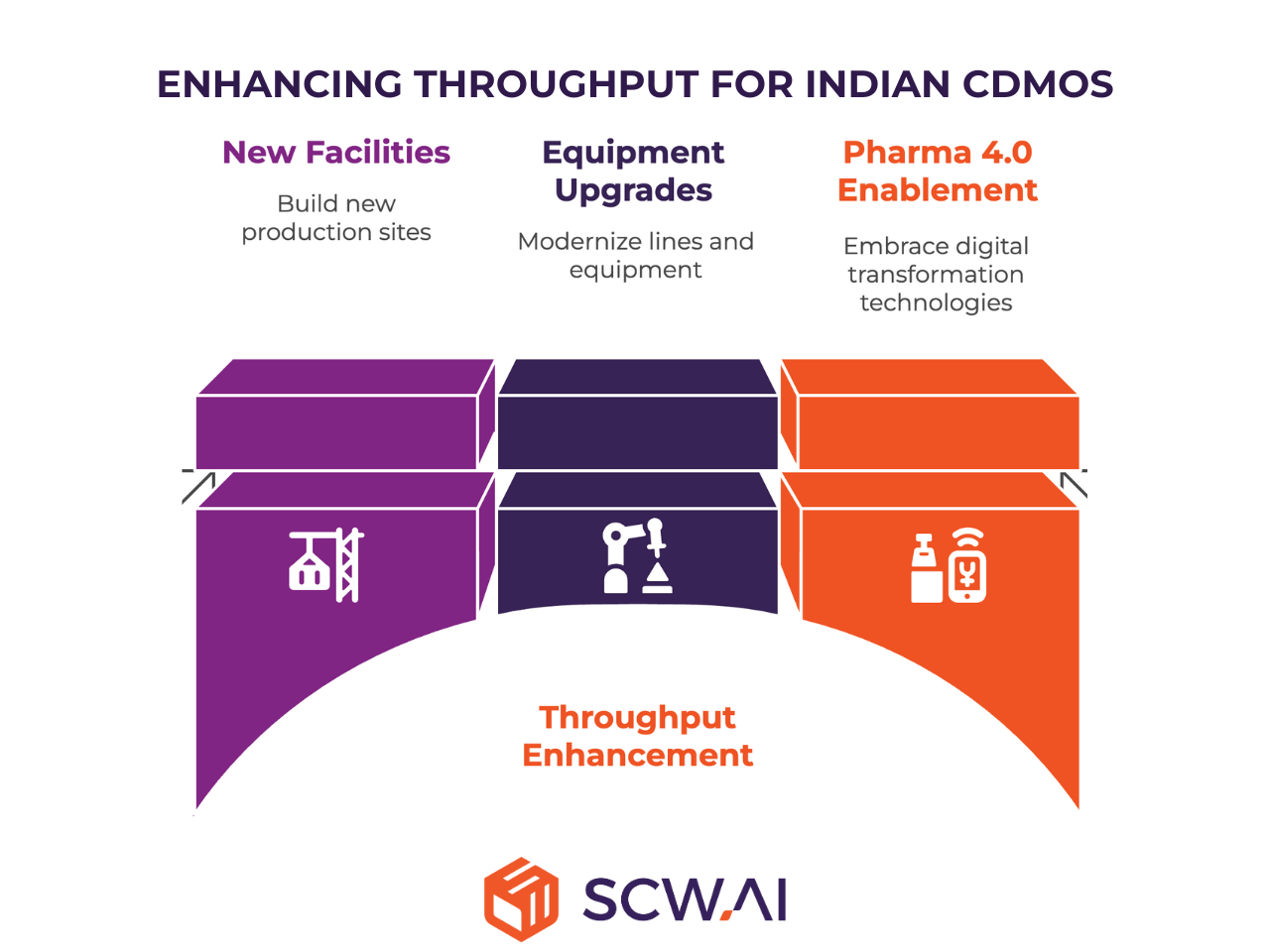
Urgent Need for Capacity Increase
The projected doubling of India’s pharmaceutical exports by 2030 and tenfold increase by mid-century, coupled with surging domestic demand, creates an immediate and pressing need for Indian CDMOs to rapidly scale manufacturing capacity. Without proactive investment in new facilities, equipment upgrades, and the strategic adoption of Pharma 4.0 technologies, the sector risks missing out on significant opportunities.
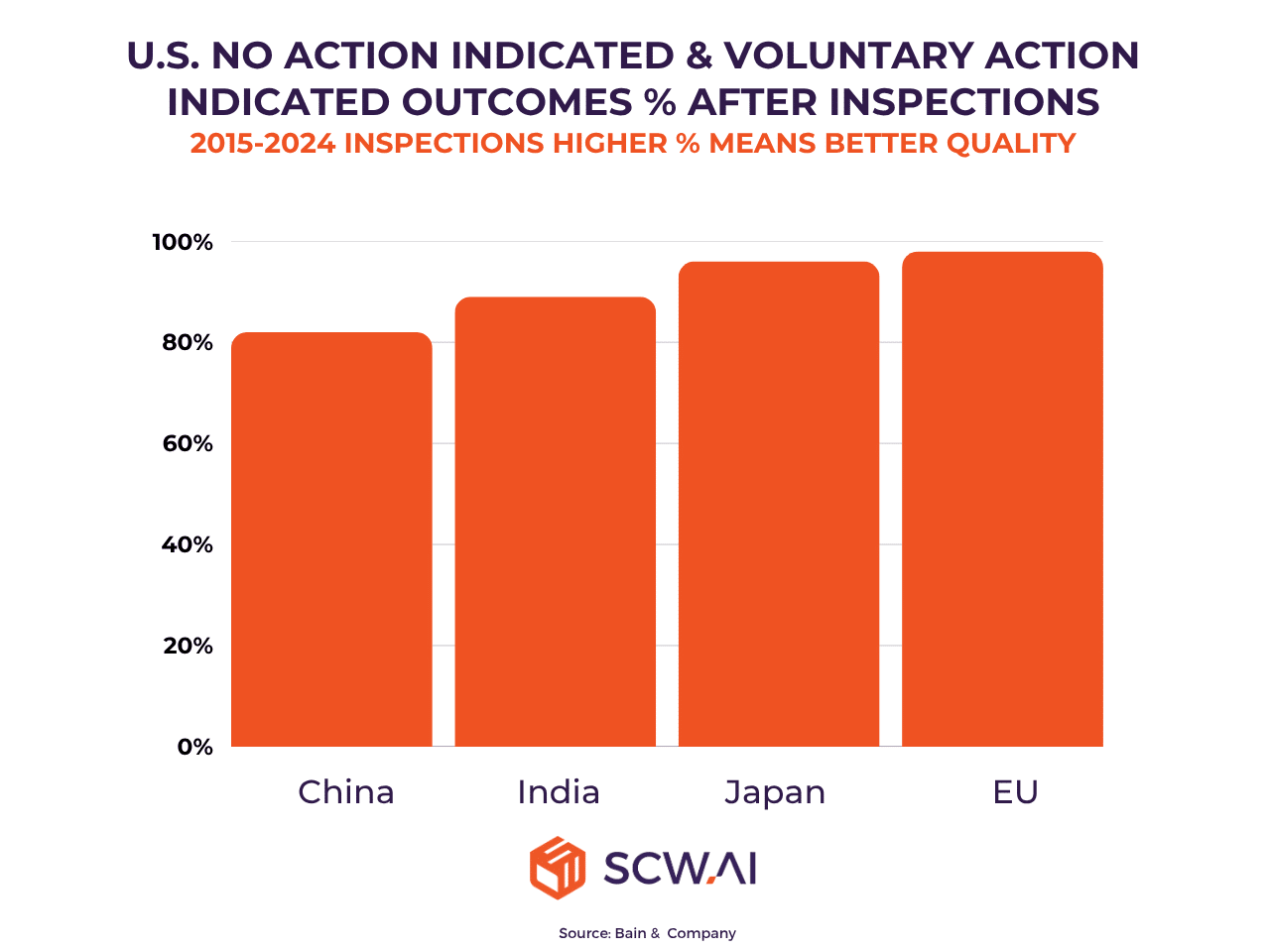
Navigating Quality Control and Regulatory Compliance
Maintaining world-class quality and adhering to stringent global regulatory standards (like FDA and EMA) remains a continuous challenge. While Indian pharmaceutical manufacturers have shown improvement in inspection outcomes, there is still a gap compared to leading global counterparts. The white paper emphasizes that achieving and sustaining top-tier quality requires a culture of continuous improvement, operational discipline, and digital rigor, moving beyond mere periodic compliance to make quality a competitive differentiator.
Impact of Evolving Global Trade Dynamics
The rise of protectionism and on-shoring trends in major markets, exemplified by the U.S. Section 232 Investigations and the EU Critical Medicines Act, poses strategic risks. These policies aim to localize production and enhance supply chain resilience, potentially leading to new tariffs, import quotas, or stricter procurement rules for non-EU countries like India. Indian CDMOs must engage in proactive trade diplomacy and align their standards to navigate these evolving global trade dynamics.

To learn more regarding how pharma manufacturers can cope with new trade paradigm download our free eBook: “Navigating Trump Tariffs: Reshoring & Capacity Maximization Tips for Pharma Manufacturers.
Future Labor Cost Considerations
As India’s economy grows, rising labor costs will inevitably challenge its traditional cost efficiency advantage. To remain competitive, Indian CDMOs must ensure that labor productivity increases at a faster pace than wages. This necessitates significant investment in workforce skill development, industry-academia collaboration, and, critically, the accelerated adoption of automation and digital tools through Pharma 4.0 to boost output per worker.
The Digital Edge: Pharma 4.0 as the Solution
Pharma 4.0 technologies are the practical solutions enabling Indian CDMOs to overcome or reduce impacts challenges and accelerate their growth. Our white paper details how these digital advancements translate into tangible benefits across the factory floor:
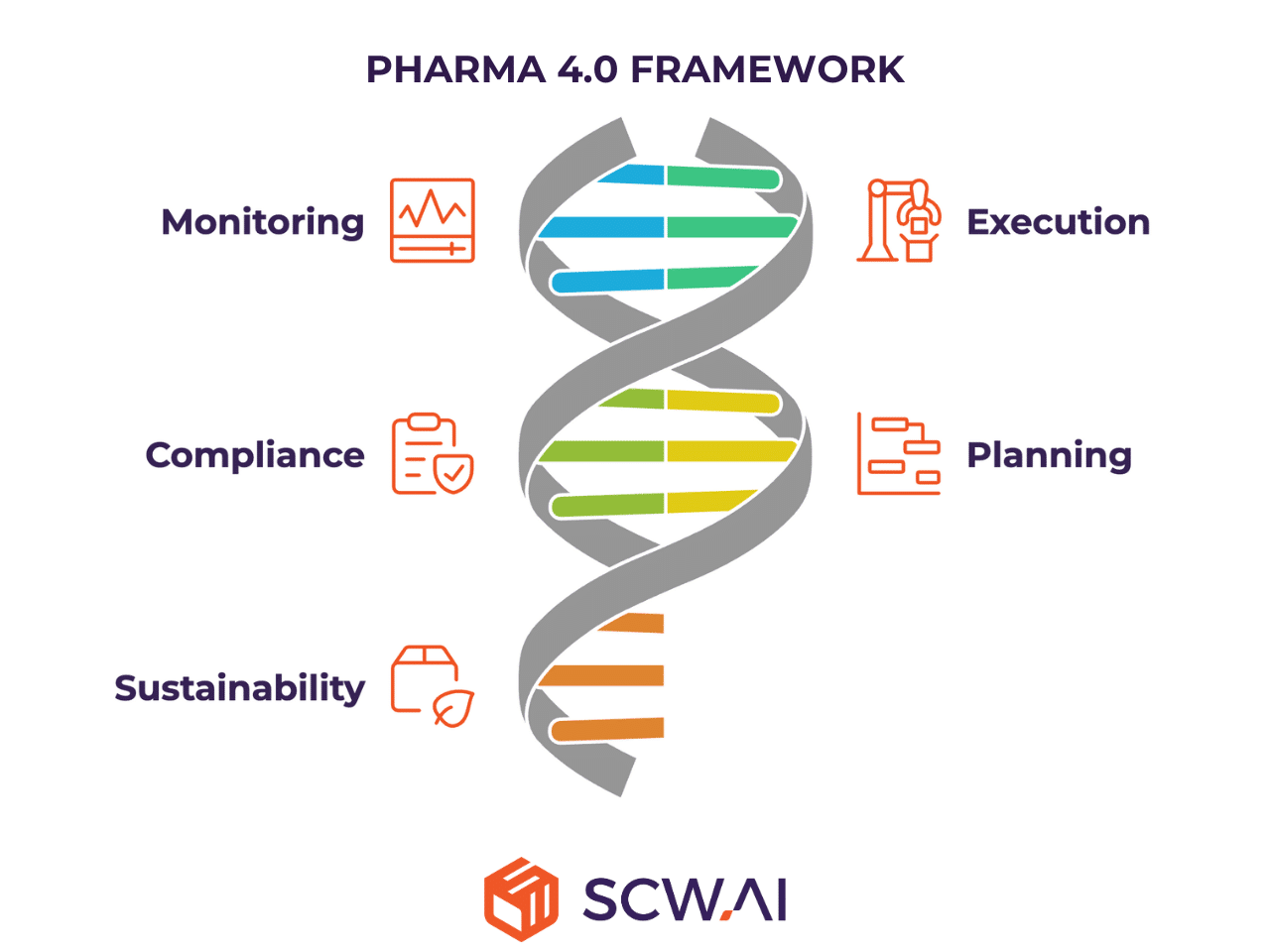
Enhanced Factory Monitoring: Digital monitoring solutions provide real-time visibility into operations, allowing for immediate identification of inefficiencies and proactive decision-making. This directly reduces costs and quality deviations, boosting overall output.
Streamlined Task Execution: Digital tools for shop floor management replace fragmented, paper-based processes with structured, accountable workflows. This accelerates continuous improvement and ensures consistent execution, crucial for maximizing throughput and maintaining quality.
Improved Regulatory Compliance through Paperless Quality: Transitioning to paperless quality systems ensures ALCOA+ data integrity, drastically reduces manual errors, and embeds compliance directly into daily operations. This is vital for achieving favorable regulatory outcomes and building customer trust.
Optimized Planning with Responsive Scheduling and Predictive Maintenance: AI-powered scheduling tools enable dynamic production planning that adapts to real-world constraints, minimizing changeovers and maximizing on-time delivery. Coupled with predictive maintenance, which anticipates equipment failures before they occur, these solutions significantly reduce downtime and enhance operational resilience.
Fostering Sustainable Manufacturing and ESG Compliance: With increasing global scrutiny on environmental impact, Carbon Trackers provide real-time visibility into emissions and their associated costs. This allows Indian CDMOs to proactively manage their carbon footprint, align with tightening ESG regulations, and enhance their market appeal as responsible global partners.
Unlock India's Pharma Future: Download the Full White Paper
This blog post offers a glimpse into the strategic insights contained within our comprehensive free white paper, “India CDMO Market White Paper: A Roadmap to Becoming a Leader in Pharma Manufacturing.”
For Indian CDMO manufacturers, top managers, pharma factory leaders, digital transformation officers, and operations managers, this white paper provides:
- Detailed data and in-depth analysis of the growth drivers and strategic advantages.
- A granular examination of the micro and macro challenges, including specific impacts and mitigation strategies.
- A practical roadmap for digitizing pharmaceutical shop floors, leveraging Pharma 4.0 technologies with actionable use cases.
- Insights to help you make informed investment decisions that will significantly improve your future readiness and competitive positioning.

Don’t miss out on the full picture. Download the complete white paper today for detailed information and a comprehensive guide to navigating India’s pharmaceutical future.
A Digital Roadmap for India CDMOs
SCW.AI’s Digital Factory Platform is a pharma-specific, modular solution built to support the diverse digitalization needs of CDMOs—ranging from oral solid dosage (OSD) and sterile injectables to biologics, biosimilars, plasma derivatives, creams, and ointments. Designed for rapid deployment, the platform offers full-scale implementation in under 100 days, ensuring a fast and cost-effective digital transformation journey.
This end-to-end, scalable platform delivers advanced capabilities across five key pillars:
- Monitoring
- Execution
- Paperless compliance
- AI-powered planning
- Sustainability
Indian CDMOs can choose to deploy individual modules tailored to their immediate needs or implement the full suite for a holistic transformation—adapting to any level of digital maturity. Whether the objective is enhancing OEE, achieving world-class quality standards, improving labor productivity, or supporting agile planning, SCW.AI equips manufacturers with the digital infrastructure needed to compete globally.
In alignment with the India@100 vision, SCW.AI partners with local experts such as Conval Group to ensure alignment with Indian pharmaceutical regulations, operational excellence standards, and industry best practices. This collaboration ensures that every transformation initiative is deeply rooted in practical experience, compliance know-how, and scalable digital technology.
To learn more about how SCW.AI can help future-proof your factory, connect with us today.
Book a demo today and explore how SCW.AI’s Digital Factory Platform can help position your organization as a global leader in pharmaceutical development and manufacturing.
Get Your Free "India Pharma CDMO Market White Paper"
Fill out the form to request your free download.