
To boost overall efficiency and profitability, pharmaceutical manufacturers need to minimize the planned and unplanned downtimes in the shop-floor. However, machines and employees cannot operate continuously, making it impossible to completely eliminate the downtimes in production. This makes it necessary to plan to minimize this period of time as much as possible, and this planning process is called “Changeover Time Reduction”.
What is Changeover Time in Manufacturing?
Changeover time refers to the process of transitioning from one batch, work order, or process to another in a production line, involving activities such as equipment adjustment, reconfiguration, and cleaning to ensure smooth production flow and meet changing requirements. On the basis of simpler terms, it is the total time elapsed between the production of the last good product of the previous run and the first good product of the following run.
Changeover time plays a crucial role in the dynamic world of pharmaceutical manufacturing, serving as a necessary part of production procedures and they are typically meticulously planned and integrated into job shop scheduling as each minute spent on changeover activities represents an availability loss in Overall Equipment Effectiveness (OEE). Therefore, it is important to optimize changeover time and complete it as efficiently as possible.
Changeover Time Example in Pharmaceutical Manufacturing
Let’s say a pharma manufacturing company produces two types of antibiotics tablets, “Bio Cure” and “Body Defend” on the same line. The changeover process involves cleaning the equipment, changing the dies (the parts that shape the tablets), and calibrating the equipment for each product. Before working on any improvement techniques, let’s assume the changeover time between the “Bio Cure” and “Body Defend” production takes approximately 3 hours, leading to delays and increased costs. To address this issue and optimize changeover time, the company can use different strategies, two of which can be:
Increasing the Number of Minor Changeovers:
Instead of waiting for a large batch of “Bio Cure” tablets to be completed before switching to “Body Defend”, the company can decide to implement more frequent, smaller changeovers. This approach involves producing smaller batches of each product before transitioning to the next one. By increasing the number of minor changeovers, the changeover time is reduced since the equipment adjustments and calibration required for each changeover become more familiar and routine for operators.
Classifying Changeovers:
The pharmaceutical company can classify changeovers into three categories based on complexity and setup/cleanup time:
Minor Changeovers: These are quick and straightforward transitions that require minimal adjustments and can be completed in a short time. For example, changing the color of the tablets or switching to a different packaging size.
Medior Changeovers: These changeovers involve moderately complex tasks such as changing the shape or imprint of the tablets. While they take longer than simple changeovers, they are still manageable with proper planning.
Major Changeovers: These are more complex and time-consuming changeovers, like introducing entirely new formulations or introducing different ingredients. Major changeovers require careful planning and coordination.
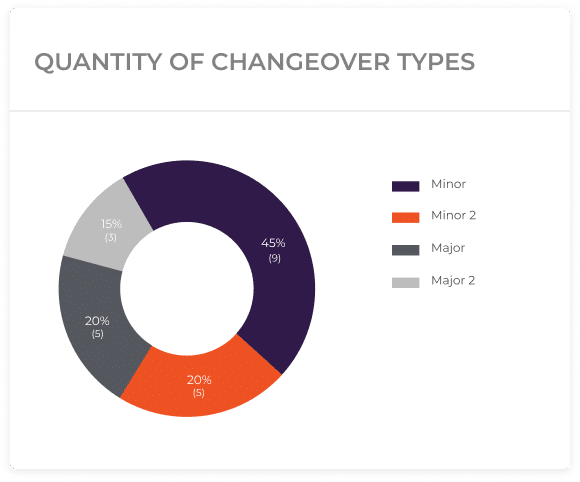
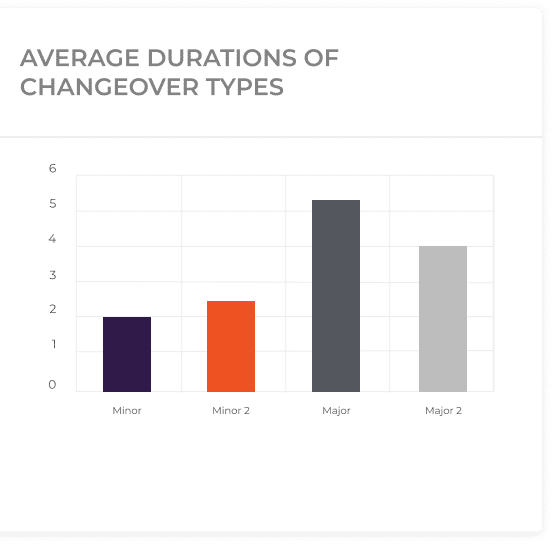
With the implementation of these two strategies, this pharmaceutical company can successfully reduce the changeover time between “Bio Cure” and “Body Defend” production, which can approximately be from 3 hours to just one hour.
What is The Traditional Industry Target for Changeover Time?
There is no universal industry target for changeover time that applies to all manufacturing sectors as it can vary widely based on the type of industry, the complexity of the manufacturing process, the size of the production run, and other factors.
Pharmaceutical manufacturing especially is known for having relatively long changeover times. This is mostly due to factors like regulatory compliance, product complexity, cross-contamination concerns, validation processes and risk mitigation. There are cases where the whole day is spent on a major cleanup of the production line. Overall, roughly 20-30% of the available time is spent on changeover activities in pharmaceutical manufacturing.
In general, manufacturing facilities that produce a wide variety of products will likely have longer changeover times compared to those producing a single product as switching between different products often involves adjusting settings and replacing tooling.
Another factor is standardization. Implementing standardized components and tooling across different products can help reduce changeover time. When setups are consistent, operators become more familiar with the process, leading to quicker transitions.
Benefits of Reducing Changeover Time in Pharmaceutical Manufacturing
As stated before, despite being a period of time that production has to stop for, changeover time is a crucial and a necessary part of pharma manufacturing. With this in mind, the omission of said time is definitely out of the question, yet condensing it into a shorter time period is the key to achieving enhanced production efficiency and many more beneficial outcomes, some of which could be:
Increased Productivity
By minimizing the changeover time and in return the overall downtime, factories can maximize the time spent on actual production or value-added activities. This leads to a much more efficient production environment as there will be more time to produce more products/services.
Enhanced Quality Control
Lengthy changeover times increase the likelihood of errors or defects during the changeover process. By reducing changeover time, organizations can maintain better quality control, ensuring consistent adherence to quality standards.
Improved Responsiveness to Customer Demand
By reducing changeover time, organizations can swiftly respond to fluctuations in customer demand. They can adjust production schedules more efficiently and switch between different production lines or variations, ensuring timely delivery and meeting customer demands effectively due to a more flexible and responsive production system.
Cost Savings
Reducing changeover time leads to a considerable reduction in cost in an organization, which is achieved through lowering setup, reconfiguration and calibration costs while also minimizing material waste during transitions.
Optimized Inventory Management
Minimizing changeover time also streamlines inventory management by allowing for smaller batch sizes and increased production frequency, leading to faster processing times which ultimately results in more revenue generation for the company.
Data-Driven Insights
Gathering accurate and comprehensive data on changeover durations provides valuable insights into the current state of operations. By analyzing this data, manufacturers can identify bottlenecks, areas of improvement, and potential sources of inefficiency during changeovers. Data-driven insights enable informed decision-making and facilitate targeted optimization efforts.
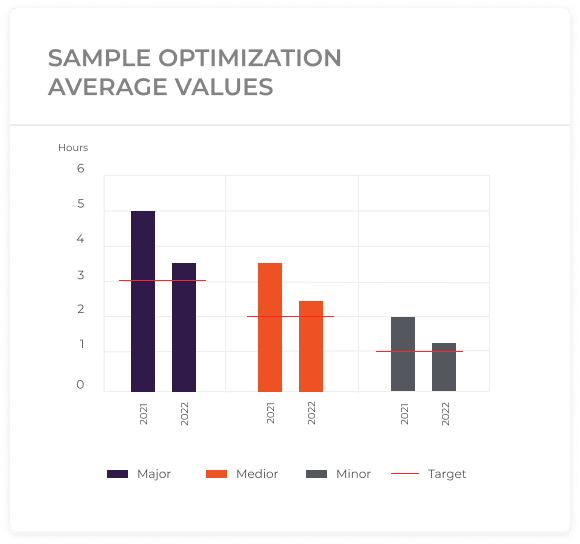
Potential Savings
By subtracting the actual changeover durations from the target durations, pharma manufacturers can calculate the time saved for each changeover instance. Summing these time savings up across multiple changeovers reveals the cumulative impact of changeover optimization. Potential savings represent additional available production time that can be utilized for manufacturing more products within the same timeframe. This, in turn, leads to increased output and revenue generation. Moreover, the saved time can be used for other cost reduction initiatives. It can be allocated to other important tasks such as equipment maintenance, process improvement, operator training, or quality control measures. By reallocating the saved time to these activities, pharma manufacturers can enhance overall operational efficiency, reduce expenses, and further optimize production processes.
Additionally, the time saved through changeover optimization contributes to reducing lead times and improving delivery schedules. With shorter changeover durations, manufacturers can be more responsive to customer demands, ensuring timely delivery and enhancing customer satisfaction. This can strengthen relationships with clients and provide a competitive advantage in the marketplace.
How to Reduce Changeover Time in Pharma Manufacturing?
Effective changeover time planning involves considering factors such as equipment availability, tooling requirements, and operator skill sets. By carefully scheduling changeovers, pharma manufacturers can optimize resource allocation, minimize setup and cleanup times, and avoid production disruptions.
Comparison with Target Values
Establishing target durations for changeover activities is vital for benchmarking performance and setting improvement goals. By comparing actual changeover durations with the predefined targets, pharma manufacturers can identify gaps and focus on areas requiring attention. This comparison serves as a performance indicator and highlights the effectiveness of changeover optimization initiatives.
Classifying Changeovers
Categorizing changeovers into minor and major types provides a framework for better planning and resource allocation. Minor changeovers involve smaller adjustments, such as product variants or limited process modifications, while major changeovers encompass significant transformations, such as complete equipment reconfiguration or product line changes. By accurately counting and classifying changeovers, manufacturers can identify patterns, prioritize improvement efforts, and optimize changeover durations accordingly.
Increasing the Number of Minor Changeovers
Efficient planning and sequencing of production orders can help increase the number of minor changeovers, leading to improved production flexibility and reduced changeover durations. By strategically grouping products or processes with similar changeover requirements, manufacturers can minimize transition times and enhance overall efficiency. This approach allows for quicker adjustments, reduces downtime, and enables better utilization of resources.
Data Driven Analysis
To reduce changeover time and optimize production processes, manufacturers can also use data-driven analysis. By examining past production data, they can identify patterns and bottlenecks in changeover durations. Comparing actual changeover times with target values provides insights into areas that require improvement. For instance, a pharmaceutical manufacturing company may discover that certain product variants consistently have longer changeover durations due to specific equipment adjustments or configuration changes. By utilizing a quicker way of adjustment or using a separate configuration tool, they can reduce changeover times significantly. This data-driven approach empowers manufacturers to make objective and informed decisions, optimize changeover processes, and enhance overall production efficiency.
Improved Changeover Planning
A well-structured planning approach ensures smooth changeovers, eliminates unnecessary downtime, and maximizes production output. By leveraging production data, comparing it with target values, and implementing better planning strategies, pharma manufacturers can achieve significant improvements in changeover durations. This data-driven approach enables them to identify opportunities for optimization, streamline operations, and enhance overall production efficiency.
SMED
Single-Minute Exchange of Die (SMED) is a concept that targets reducing changeover time by identifying and eliminating inefficiencies during the operation. Its main goal is to minimize downtime during the transition process. By distinguishing between internal and external changeover activities, SMED methodology targets tasks that can be performed while the equipment is still running or completed in advance. This approach optimizes equipment availability, as operators can focus on setup tasks without interrupting the ongoing production which eventually results in higher productivity, reduced costs, and improved customer satisfaction.
Conclusion
Gathering production data, comparing changeover durations with target values, and implementing effective planning strategies are critical components of changeover optimization. By utilizing data-driven insights and focusing on minor changeovers, pharma manufacturers can minimize downtime, increase production flexibility, and drive continuous improvement. Embracing these practices empowers manufacturers to optimize changeover durations and achieve enhanced operational efficiency, ultimately contributing to improved Overall Equipment Effectiveness (OEE) and overall business success.
To see how our Changeover Analysis and other cloud manufacturing solutions can assist you in reducing your changeover time in your shop-floor, Book A Demo now!


