Pharmaceutical manufacturers left money on the table! Our analysis integrates insights from a McKinsey study, delving into how pharmaceutical manufacturers can improve many aspects of their Overall Equipment Efficiency (OEE) through strategic digital transformation investments. Utilizing the SCW.AI ROI calculator, we demonstrate how enhancements in OEE can amplify throughput and annual returns. The findings emphasize that directing investments towards optimizing changeover time holds the capacity to elevate overall OEE by 3 to 6 points. This, in turn, corresponds to an approximate 2.5% to 5% surge in throughput for an average pharmaceutical manufacturer.
In this article, we provide a detailed introduction to our Changeover Analysis Report and how it can help pharmaceutical executives realize these commercial benefits. We also highlight its main features and describe how it can help pharmaceutical manufacturers reduce changeover times significantly. Finally, an appendix at the end of this article details our methodology.
Potential Business Benefits of SCW.AI’s Changeover Analysis
To systematically clarify the benefits of Changeover Analysis for producers of pharmaceuticals, we investigate three different factory sizes: small, medium, and large. We also present two scenarios to illustrate distinct possibilities for OEE improvement. Based on our experience, changeover activities can account for 15% to 30% of the total OEE. Pharmaceutical managers thus have the chance to improve their OEE by 3 to 6 points.
The below image illustrates potential business benefits of our Changeover Analysis:

Note: These figures are estimated, which has some potential limitations and is dependent on a variety of different assumptions (including definition of small, medium-sized and large factories). Read our Appendix at the end of the post to find out more!
To learn more regarding changeover time definition and its best practices you can read our How to Reduce Changeover Time in Pharma Manufacturing article.
Changeover Analysis Offers Granular Data to Minimize C/O Time
For pharmaceutical manufacturers, changeovers are a necessary but time-consuming part of the production process. Companies are audited by agencies such as the FDA to verify their compliance with procedures aimed at safeguarding public health. One critical requirement is that production lines must be thoroughly cleaned after each work order.
The amount of time required for cleanup increases if there is a difference in the drug class between two work orders. In addition, setting up machines for new products can be a time-consuming process. Other problems, such as ineffective planning, can also contribute to non-optimal changeover time (c/o time).

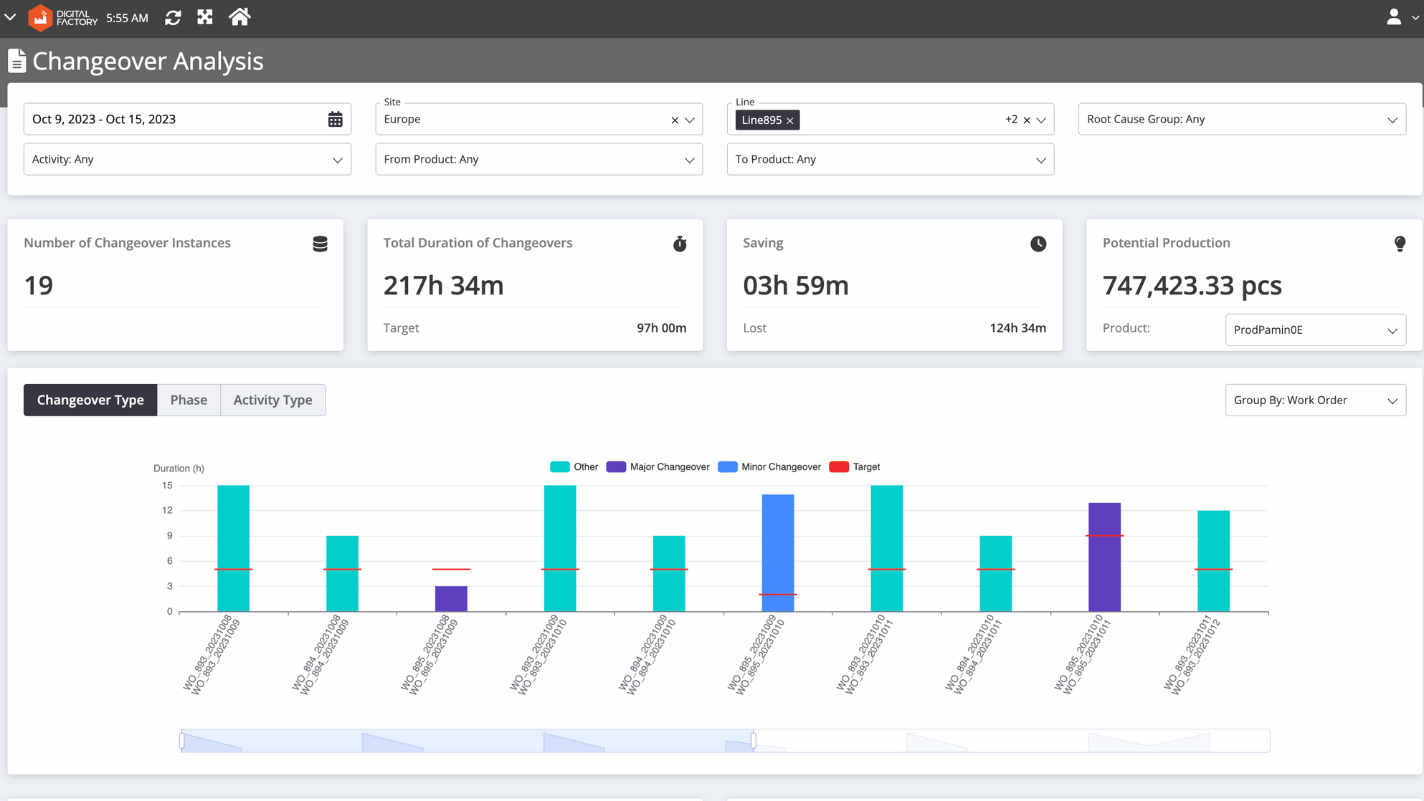
To reduce changeover time, pharmaceutical executives need granular data that gives them precise information about where bottlenecks are occurring. SCW.AI’s Changeover Analysis provides the following data to help executives take appropriate action:
- Average Durations of Changeover Types: This metric shows how much time is spent on different types of changeover activities, such as line cleaning, machine set up, and configuration. Executives can use this information to identify areas for improvement in general changeover procedures.
- Quantity of Changeover Types: Increasing the number of minor cleanups instead of more major ones can lead to better production planning and shorter c/o time. Managers should track the quantity of changeover types in order to implement and track this strategy.
- Product-Based Changeover Data: It is possible that the production of specific work orders, products or product families is causing changeover inefficiencies. Executives need product-based changeover data to determine whether this is the case.
- Line-Based Changeover Data: Certain production lines may suffer from more unplanned downtime or idle time than others. Companies need line-based changeover data to identify and address the root causes of these problems.
- Activity-Based Changeover Data: The pharmaceutical manufacturing process involves a variety of production steps, such as mixing, blending, granulation, compression, encapsulation, coating, printing, inspection and packaging. Administrators need activity-based changeover data to determine which tasks take the longest to complete or require the most resources.
- Deviation From Planned Changeover Time: Effective planning requires close alignment between planned and actual changeover times. If c/o time is less than scheduled, companies are missing out on opportunities to produce more products. On the other hand, actual changeover times that exceed scheduled time frames can be a sign of inefficient utilization of labor, capital, or both. Managers can use Changeover Analysis to track deviations from planned c/o time.
- Potential Production: The Potential Production metric shows how many more products you could produce if there was no deviation from planned changeover time.
- Time Frame Selection Capability: Managers can check any of the KPIs we specified above within a particular time window.This allows you to track your progress toward achieving your goals.
3 Ways to Utilize Changeover Analysis
SCW.AI’s Changeover Analysis can be used in three main ways to help pharmaceutical manufacturers identify bottlenecks and increase throughput.
1. Find Out Your Deficiencies
Unplanned downtimes and idle periods can have a significant impact on productivity, leading to losses in production output and revenue. These problems are often the result of underutilized human and capital resources, such as personnel who need more training or machinery that need maintenance. Granular data from Changeover Analysis can be used to identify the underlying reasons for long changeover times.

2. Optimize Planning by Decreasing the Number of Major Changeovers
By strategically scheduling production with similar raw materials, companies can reduce the need for extensive clean-ups and long changeover periods (planned downtimes). This approach empowers planning managers to assess the effectiveness of their schedules and make data-driven decisions. KPIs related to the frequency of minor changes can be established, allowing companies to progressively enhance their operations over time.
This strategy can lead to a noticeable reduction in changeover times in the long run, ultimately boosting overall efficiency in the production process.
3. Reduce Unplanned Downtimes With Data Analysis
Changeover Analysis enables businesses to monitor and analyze equipment performance in real-time, allowing them to detect anomalies and early warning signs of potential issues. This predictive maintenance approach helps companies foresee when equipment may require maintenance or replacement, reducing the likelihood of sudden failures.
To witness all capabilities and benefits of Changeover Analysis and discover more about Factory of Future You can Schedule a Demo Now!
Appendix
From McKinsey’s study on pharmaceutical manufacturers, we extracted the average OEE of pharmaceutical companies and the possible OEE benefits by implementing steps like cutting brief stops or lowering c/o time via digitalization. We enriched this information with our data to create different scenarios and improve representation of our benchmarking.
To find out throughput and annual return increase we used our ROI Calculator. To run the analysis, we filled the following inputs in the given way:
- ROI Strategy: Allocation of the improvement in productivity between reduced labor expenses and higher capacity/throughput (more profits). We picked a 30% increase for production volume.
- Production Days: We assumed 250 working days per year.
- Production Hours per Day: We assumed 16 working hours per day.
- Number of Production Lines: Represents the manufacturing capacity of a pharmaceutical factory. To illustrate the influence of Changeover Analysis on annual return for small, medium-sized, and large factories, we selected three alternative scenarios: 10, 50, and 100.
- Average Operators per Line: We assumed each line had five operators working.
- Hourly wage: The average cost of an operator in the US is ≅$20 per hour, therefore that is what we chose.
- Annual Revenue: To represent small, medium-sized and large factories we selected three alternative scenarios as $10M, $50M and $100M revenues.
- Current OEE: We selected 35, which McKinsey estimated to be the average OEE of pharmaceutical firms. Our case studies show that the industry average is similar.
- Target OEE: Calculated by adding the existing OEE to the potential OEE enhancement through digital transformation in specific operations. McKinsey identified changeover as constituting 15% of the total OEE and projected that digitizing changeover time could yield a 3-point OEE improvement. However, our customer data indicates that changeovers may account for up to 30% of OEE, presenting an opportunity for a 6-point OEE enhancement. Consequently, we consider two scenarios: in the first, the target OEE is 35 + 3 = 38, and in the second, it is 35 + 6 = 41.
- Gross Margin: Taken as 40% for all scenarios.
For more data-driven insights for the changeover durations in pharma you can contact us.