
GMP Compliant Paperless Manufacturing Solution for Regulated Industries
The Digital Logbook is your GMP-compliant solution for entering, monitoring, reporting, and auditing production forms and logs. Designed with insights from industry experts, it ensures log entries and storage are faster, more secure, and more accurate compared to traditional paper-based methods. Adhering to ALCOA+ data integrity principles, the Digital Logbook ensures compliance with FDA and EMA regulations, protecting manufacturers from potential non-compliance penalties.
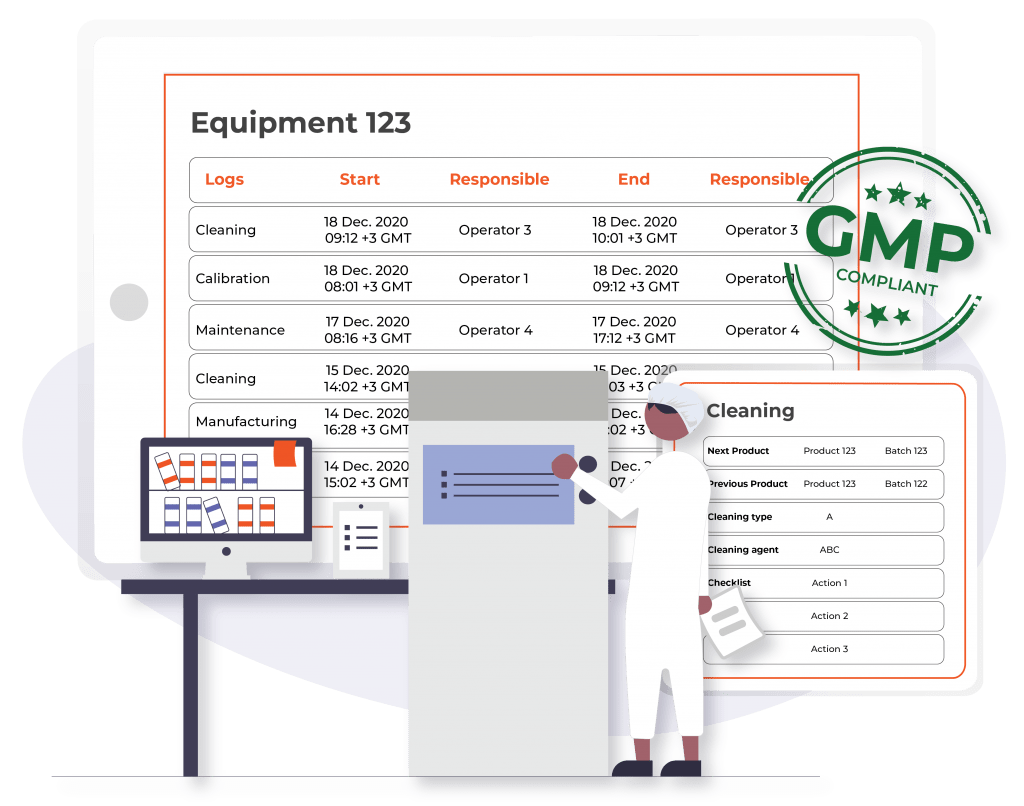
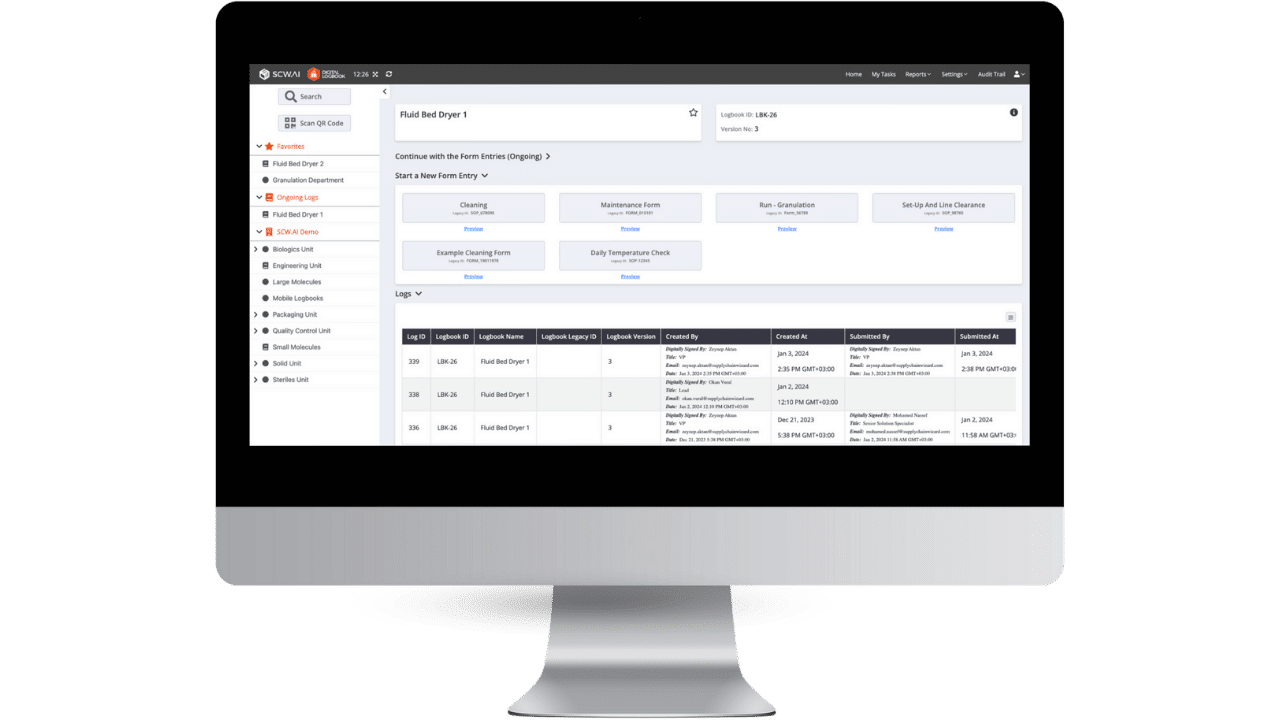
Top Use Cases of Digital Logbook
- Enhanced Regulatory Compliance: Ensures adherence to industry regulations and standards, such as FDA 21 CFR Part 11 and EMA guidelines. Facilitates the audit process by allowing users to easily check any specific log entry, reducing the risk of non-compliance penalties.
- Increased Data Analysis Capability: Transforms factory data from archived paper logs to accessible digital logs, enabling users to conduct comprehensive analyses to identify bottlenecks and improve processes.
- Support Sustainability Initiatives: Eliminates the need for paper, helping manufacturers improve environmental KPIs such as carbon footprint, waste production, and water consumption.
- Attract and Retain Talented Graduates: Addresses the labor shortage in manufacturing by offering a digitalized work environment. Young professionals prefer modern, technologically advanced workplaces, as highlighted by a Forbes article.
Key Features of of Digital Logbook
- Digital Signatures and Time Stamping: Ensures the authenticity and traceability of records, making it easy to verify and audit data.
- Role Management: Provides tailored authorization to different roles, ensuring that users see only the data they need to complete their tasks, maintaining segregation of duties.
- Built-in Document Management System: Organizes and stores documents efficiently, making it simple to manage and retrieve necessary files.
- Full Audit Trail: Tracks all changes and actions within the system, providing a comprehensive log for auditing purposes.
- Out of Specification Notification: Alerts users to any data that falls outside predefined parameters, enabling prompt corrective actions.
- Automated Calculations and Conditional Workflows: Streamlines processes by automatically performing calculations and triggering workflows based on specific conditions.
- Automatic Data Capturing: Collects data directly from IoT devices and existing MES, ERP and LIMS systems, reducing manual entry errors and saving time.
- Uploading Documents or Links to References: Allows users to upload relevant documents or insert links to references, ensuring all necessary information is easily accessible for users.
- Real-Time Notifications: Provides immediate alerts regarding bottlenecks, completed tasks, or tasks awaiting approval, thereby improving work order management.

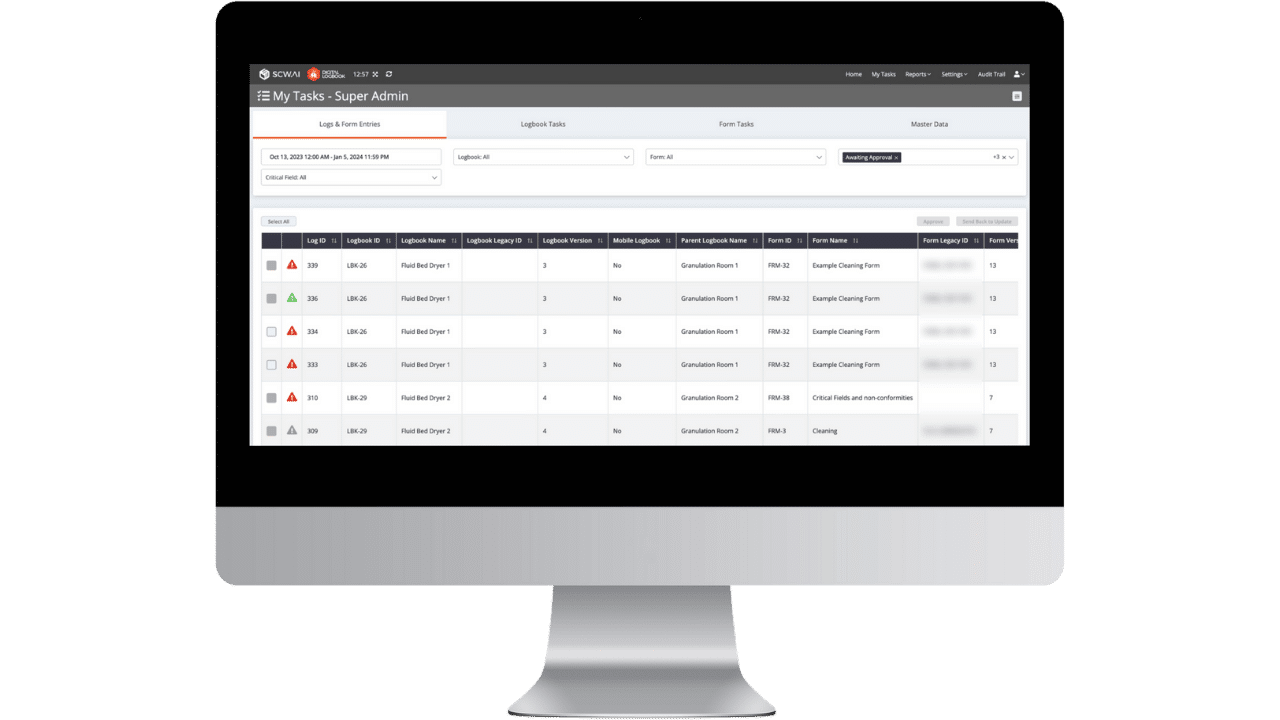
Benefits of Digital Logbook
- Reduce Risk of Receiving Warning Letters: In 2023, 6% of FDA warning letters were issued due to non-adherence to FDA 21 CFR Part 11 for log entries. By utilizing the Digital Logbook, manufacturers in pharmaceutical, food and beverage, cosmetics, and other cGMP regulated industries significantly improve their compliance.
- Minimize Data Entry Time: With smart form features and integration with existing digital infrastructure, the Digital Logbook reduces data entry time by 50% to 85%, allowing personnel to focus on value-added tasks.
- Improve Work Order Management: Slow approval of paperwork can halt production like unplanned downtime. The Digital Logbook sends real-time notifications about pending approvals, ensuring that scheduling processes are not affected by late paperwork approval.
- Cost Savings: Minimizes the expenses associated with paper-based systems, leading to significant cost reductions.
- Environmental Contribution: Each year, paper production causes deforestation of 110,000 square kilometers, an area larger than South Korea or South Carolina. On top of this, considering water consumption and CO2 emissions in paper production, switching to the Digital Logbook can be a crucial part of your green initiatives.
What Our Customers Think About Digital Logbook
“Digital Logbook has significantly improved our ability to enter and manage log entries. Now, archiving a log entry only takes 12 clicks, compared to the days it used to take. Thanks to Digital Logbook, we have reduced data entry and reporting time by approximately 85%.”
Quality Assurance Manager
Global CDMO
(USA)
(USA)
“Digital Logbook provides much clearer, more detailed steps compared to a paper logbook template—you always know exactly what you're doing."
Compression Operator
Pharma Manufacturing
(USA)
(USA)
“SCW.AI's paperless manufacturing solutions supported us in reducing waste and helped us meet our corporate sustainability targets.”
COO
Composite Technologies
(Türkiye)
(Türkiye)
“Big win for reverse traceability”
Quality Engineer Manager
Pharma Manufacturing
(USA)
(USA)


