
Strategic Questions for a More Profitable and Efficient Pharmaceutical Manufacturing Process
The pharmaceutical manufacturing industry faces increasing pressure to enhance efficiency, compliance, workforce management, and sustainability while maintaining profitability. As the landscape continues to evolve with pharma 4.0 technologies, regulatory changes, and shifting market demands, success depends on asking the right questions and making informed, data-driven decisions.
This comprehensive eBook presents seven critical questions that pharmaceutical manufacturing leaders should consider to optimize operations, reduce costs, and drive efficiency in 2025 and beyond. Each question highlights a key industry challenge and offers insights into advanced solutions, including AI-driven automation, digital transformation, and manufacturing analytics.
Who Should Read This eBook?
This eBook is designed for pharmaceutical manufacturing decision-makers, including:
- CEOs, CFOs, VPs, and Business Executives: Insights into strategic cost optimization and long-term profitability.
- CTOs and Digital Transformation Leaders: Applications of AI, automation, and real-time data for enhanced decision-making.
- Operations Heads and Plant Managers: Strategies for improving scheduling, labor management, and production efficiency.
- Regulatory and Compliance Executives: Approaches to meeting evolving cGMP and sustainability compliance requirements.
- Consultants and Industry Analysts: A data-driven perspective on emerging trends and best practices in pharmaceutical manufacturing.
Key Topics Covered in This eBook
This guide is structured around seven essential questions, each addressing a specific industry challenge:
1. How Fast Are We Identifying And Resolving Shop Floor Issues?
In pharmaceutical manufacturing, operational issues are inevitable, but the speed and efficiency of identifying and resolving them are directly linked to the digital maturity of a facility. A well-integrated digital ecosystem—encompassing real-time data gathering, automated alerts, dynamic dashboards, manufacturing analytics, and manufacturing management software (Action Tracker)—is essential for minimizing disruptions. Without these tools, issue resolution remains slow and reactive, leading to lost productivity, increased operational costs, and inefficiencies.

2. How Big is the Gap Between Paid versus Productive Labor Hours?
Pharmaceutical labor issues create a self-perpetuating crisis. Inefficient manufacturing, with 30-50% of paid hours wasted as “shadow time,” necessitates weekend and holiday work. This reliance on overtime fuels employee burnout, triggering job changes and talent loss. The resulting skill gaps and labor shortages further slow production, restarting the cycle. This downward spiral is driven by a fundamental lack of visibility stemming from inadequate digital infrastructure.
Furthermore, pharmaceutical manufacturers should improve their OEE (Overall Equipment Effectiveness) and TEEP (Total Effective Equipment Performance) to reduce overtime labor costs, as both metrics typically reveal significant time losses across various medicine production processes for the average manufacturer.
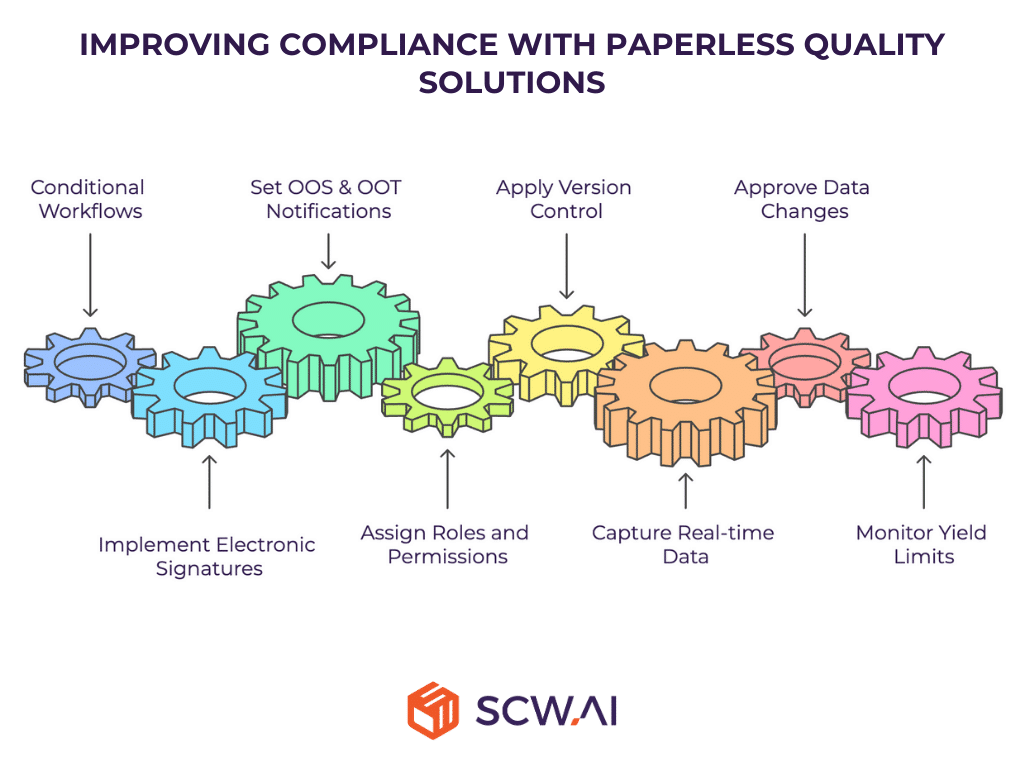
3. What Is the Value of Paperless Manufacturing in Improving Compliance?
Non-compliance with the FDA, EMA, and other regulatory bodies can result in significant penalties, product recalls, and reputational damage. Paperless quality solutions enhance compliance and reduce the likelihood of regulatory infractions. They also free up labor time previously spent on cumbersome reporting tasks and reduce the environmental impact of pharmaceutical manufacturing.
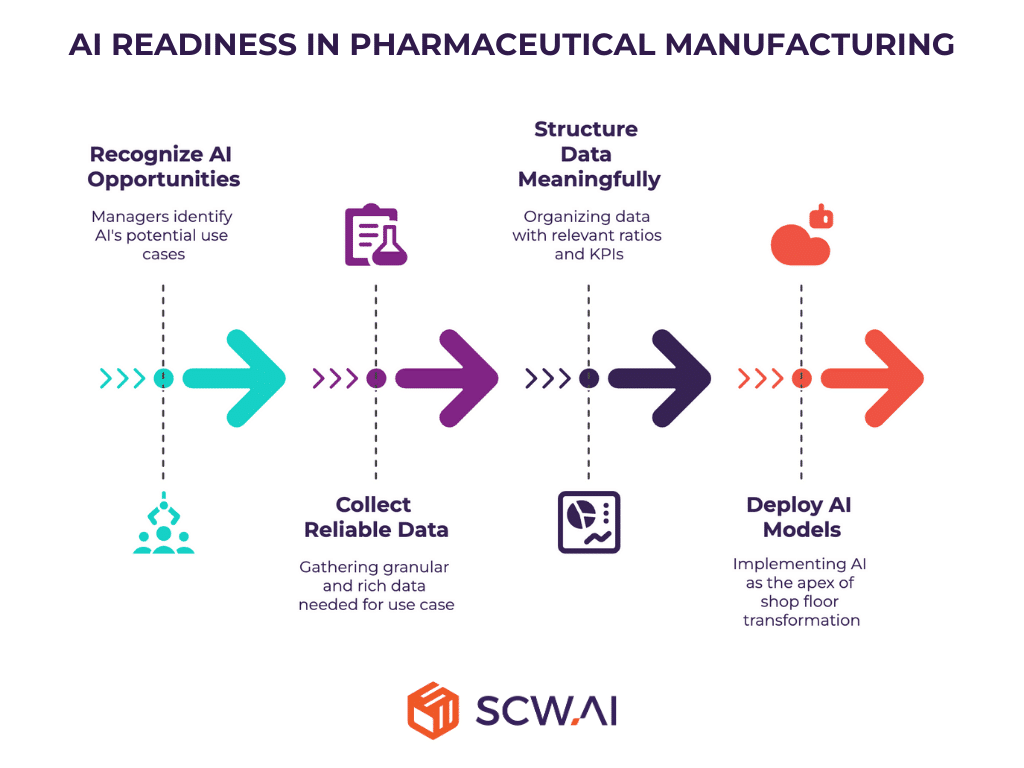
4. How Ready Are We for AI to Automate Our Operations?
AI presents lucrative opportunities for pharmaceutical manufacturers, and many managers recognize the importance of early adoption. However, mere interest in AI is insufficient for effective deployment. Digital transformation can be visualized as a pyramid: at the base, manufacturers collect reliable, granular data; the second level structures this data into meaningful metrics such as KPIs; and at the apex, AI models operate, their efficiency dependent on the quality of underlying data.
End-to-end digitalization among pharmaceutical manufacturers is approximately 2%. In such an environment, it is likely that weak digital infrastructure significantly constrains AI deployment. Additionally, organizational mindset and culture can hinder intelligent automation initiatives.
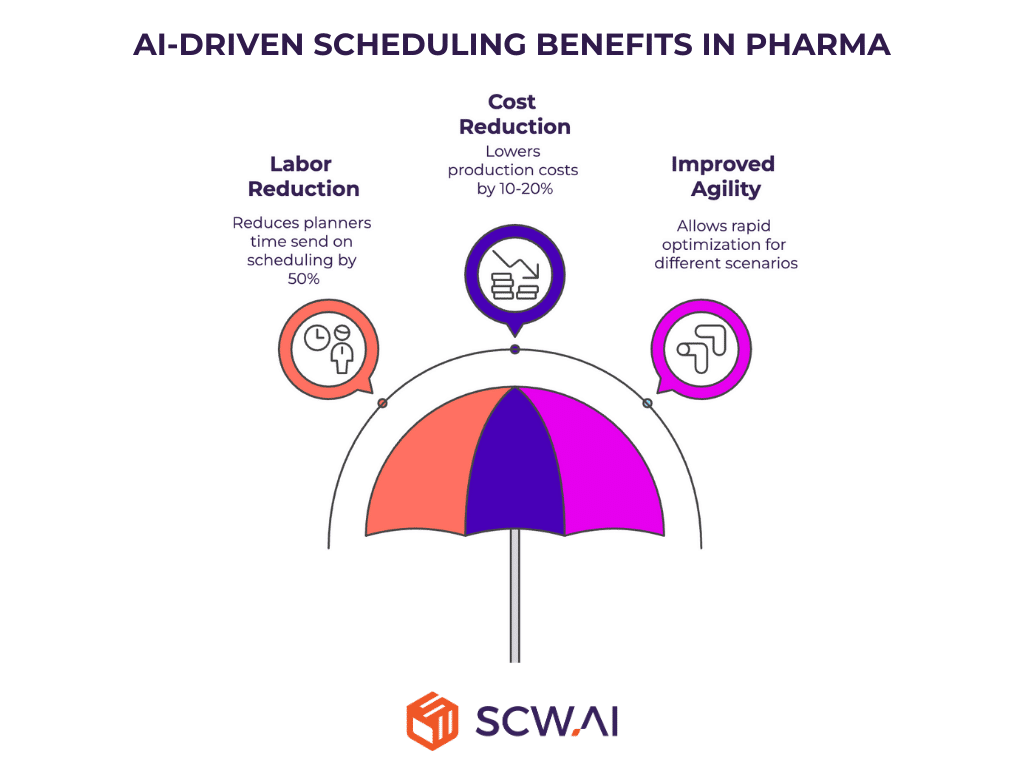
5. How Much Profit Is Lost Due to Inefficient Scheduling?
Suboptimal scheduling increases production costs, delays work orders, and limits profitability. AI-powered schedulers optimize resource allocation, minimize delays, and improve On-Time In-Full (OTIF) performance, reducing scheduling costs by up to 20%.
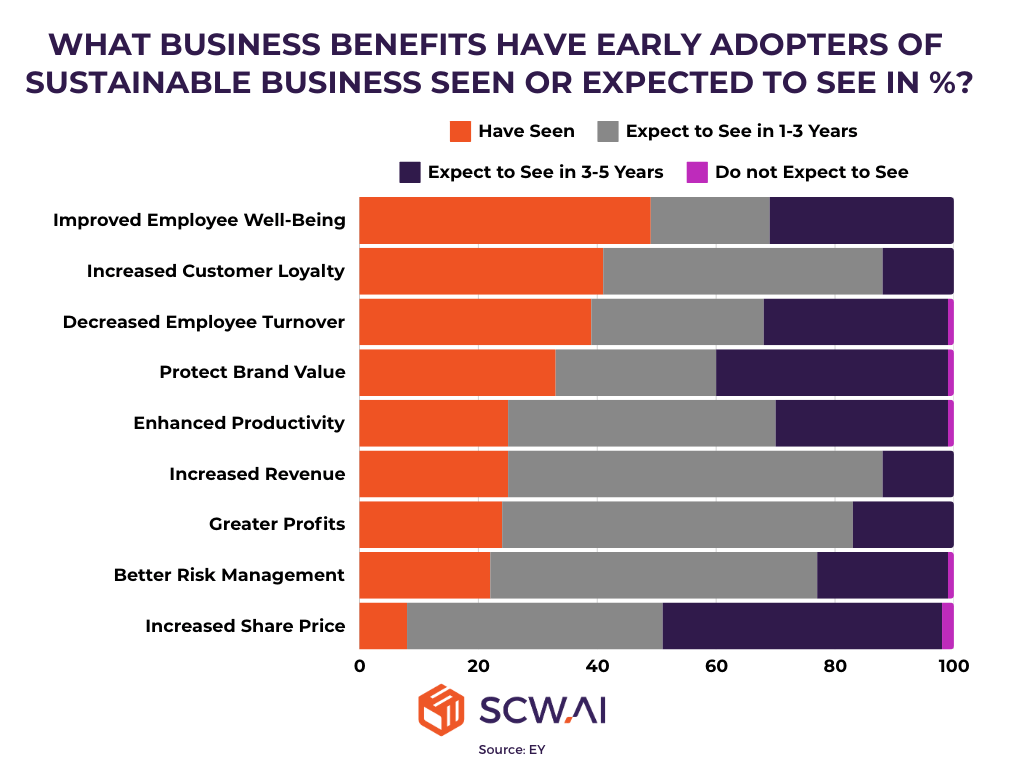
6. What is The Return of Sustainability Investments vs Risk of Delaying?
ESG compliance requirements are shaping the future of pharmaceutical manufacturing. Automating ESG reporting, tracking carbon emissions, and implementing sustainable production practices will be critical for compliance and long-term business success.
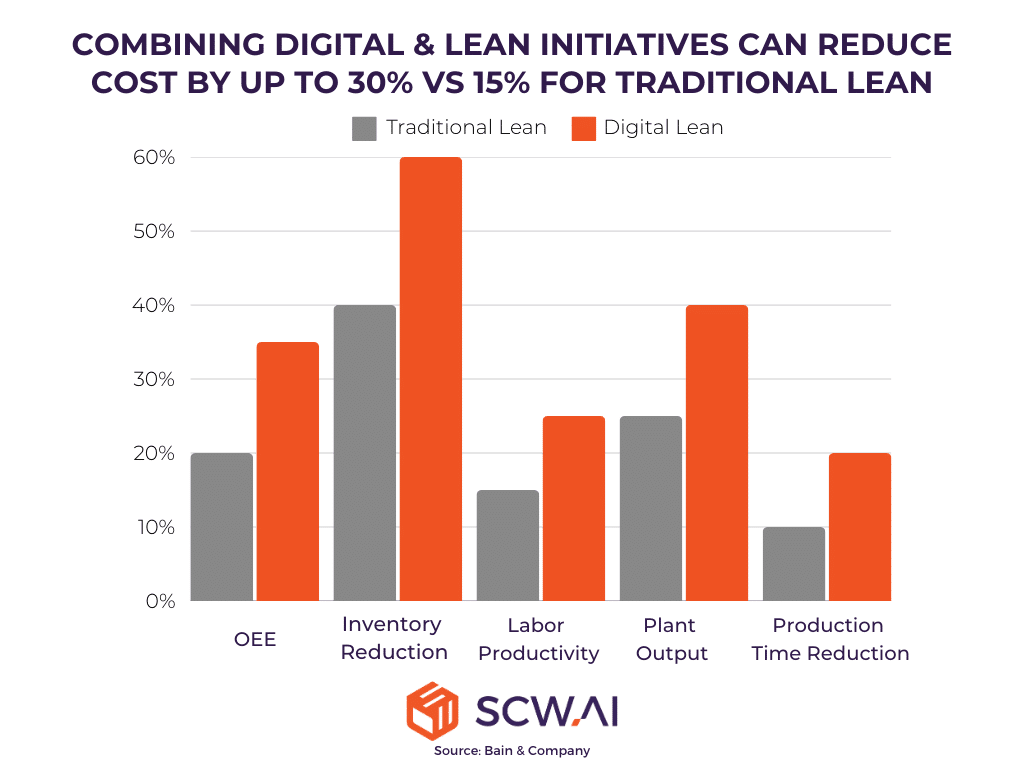
7. Does It Make Sense to Continue Lean Initiatives Without Digital Tools?
Traditional Lean Six Sigma strategies have been effective in reducing waste and improving efficiency, but digital transformation can double cost reductions—up to 30% savings. AI applications in the pharma shop floors, real-time factory visibility, and predictive analytics provide new opportunities for lean optimization.
Key Benefits of This eBook
This resource provides data-driven insights and actionable strategies for pharmaceutical manufacturers seeking to:
- Optimize operational efficiency
- Reduce costs
- Increase Throughput
- Enhance FDA/EMA compliance
- Strengthen workforce management
- Improve corporate sustainability
By thinking over these seven key questions, and reading the article, manufacturing leaders can gain a clear roadmap for improving efficiency, reducing risk, and maximizing profitability.
Download the eBook: A Strategic Guide for 2025 and Beyond

This eBook serves as a comprehensive guide to navigating the complexities of modern pharmaceutical manufacturing, providing insights, case studies, and best practices for driving efficiency and sustained profitability.
Download the free eBook today to access in-depth analysis and expert recommendations for maximizing operational success in 2025 and beyond.
Transform Pharmaceutical Manufacturing With SCW.AI’s Digital Factory Platform
At SCW.AI, our mission is to improve global well-being by making medicines more accessible and affordable. Achieving this requires enhancing pharmaceutical manufacturing efficiency through digital transformation. SCW.AI empowers manufacturers with an end-to-end Digital Factory Platform designed to reduce costs, enhance compliance, and drive world class manufacturing excellence.
Our solutions address key pain points in pharmaceutical manufacturing, including:
- End-to-End Visibility: Quickly identify and resolve bottlenecks.
- Optimized Labor Productivity: Eliminate unnecessary overtime.
- GMP-Compliant Quality Management: Reduce the risk of warning letters.
- Smart Scheduling: Increase resilience while reducing costs.
Built for scalability and ease of implementation, our platform seamlessly integrates ERPs, manufacturing execution systems, and other digital infrastructure—all deployed in less than 100 days.
For pharmaceutical producers, digitization is no longer optional—it is a strategic imperative. Book a demo today to explore how SCW.AI’s Digital Factory Platform can help you to build the pharma factory of the future.
Frequently Asked Questions
Can policymakers in the pharmaceutical industry benefit from this eBook?
This ebook offers valuable perspectives on digital transformation, regulatory hurdles, and efficiency gains within pharmaceutical manufacturing, making it a useful resource for shaping both policy and industry strategy.
I am in the early stages of my career in the pharmaceutical industry. Should I read this eBook?
Yes, it offers a holistic perspective on pharmaceutical manufacturing, making it a useful resource for professionals looking to understand industry challenges, strategies, and future opportunities.
Is this eBook about 2025 trends in pharmaceutical manufacturing?
No, it goes beyond simply addressing pharma industry trends for 2025. While it incorporates current industry data and challenges, it also offers practical strategies, case studies, and real-world Pharma 4.0 applications to facilitate long-term transformation.
Does this eBook contain technical content, or is it suitable for business leaders?
The content is designed for business leaders, operations managers, and technical professionals, presenting strategic insights with practical applications rather than highly technical content. While it inevitably contains some technical content.
How can I implement the strategies discussed in the eBook?
Many of the digital transformation and automation solutions outlined in the eBook can be deployed using platforms like SCW.AI’s Digital Factory solutions, which enable fast and scalable implementation.
Is the eBook free?
Yes, the eBook is completely free. SCW.AI is committed to providing comprehensive, up-to-date information to support the pharmaceutical industry. As a consultant and technology provider with over a decade of experience serving pharma manufacturers across five continents, we believe in sharing knowledge and giving back to the industry to drive continuous improvement and innovation.
Where can I download the eBook?
Fill out the form at the end of the page to access the full document. You can reach the form by clicking the orange “Get” or “Download” buttons or “here”. After submitting the form, you will receive an email with a link to download the document.
Are there additional ebooks or white papers available for in-depth learning for pharmaceutical professionals?
Yes. Recommended resources include:
For inquiries about SCW.AI’s Digital Factory Platform, partnership opportunities, questions about the eBook or further resources, contact our team.
Get Your Free "7 Provocative Questions Pharmaceutical Manufacturing Leaders Should Ask eBook"
Fill out the form to request your free download.