According to the World Economic Forum, the pharmaceutical industry generates over 1.5 times more carbon emissions per million dollars of revenue than the automotive sector, making it one of the most carbon-intensive industries. Ironically, while the industry is dedicated to improving human health, it also significantly contributes to climate change—the most concerning global threat for the near future.
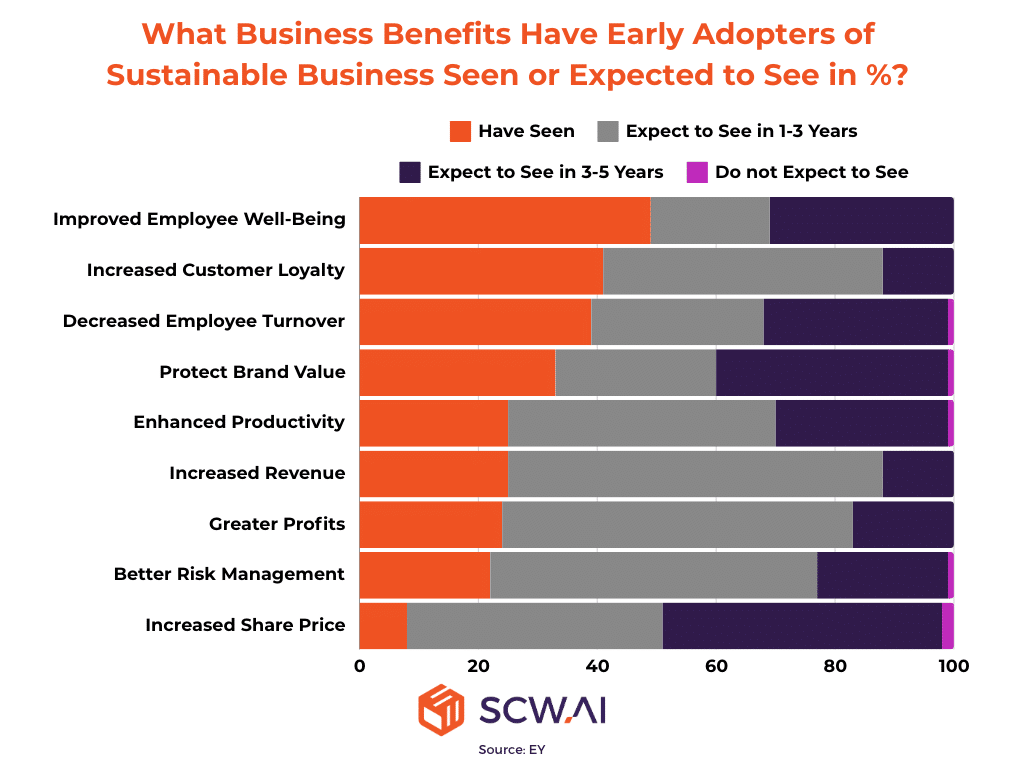
In a landscape where sustainability regulations are tightening, and consumers, investors, and business partners increasingly favor eco-friendly businesses (See Figure Below), this poses a substantial risk to the industry’s long-term profitability. This article delves into practical strategies for ensuring pharma sustainability to gain long-term competitive advantage. We will explore the latest trends, key obstacles, and best practices in sustainable pharmaceutical manufacturing. Finally, we will highlight how SCW.AI is empowering pharma manufacturers to achieve their sustainability goals.
Trends Shaping Pharma Sustainability
1. Reducing Carbon Emissions
In 2015, the Paris Agreement—signed by 196 countries—set a global target to achieve net zero emissions by 2050, aiming to mitigate the catastrophic impacts of climate change. In response, many sectors, including the pharmaceutical industry, committed to ambitious carbon reduction goals. According to a IQVIA report, the pharmaceutical sector is making progress, particularly in reducing Scope 1 and Scope 2 emissions.
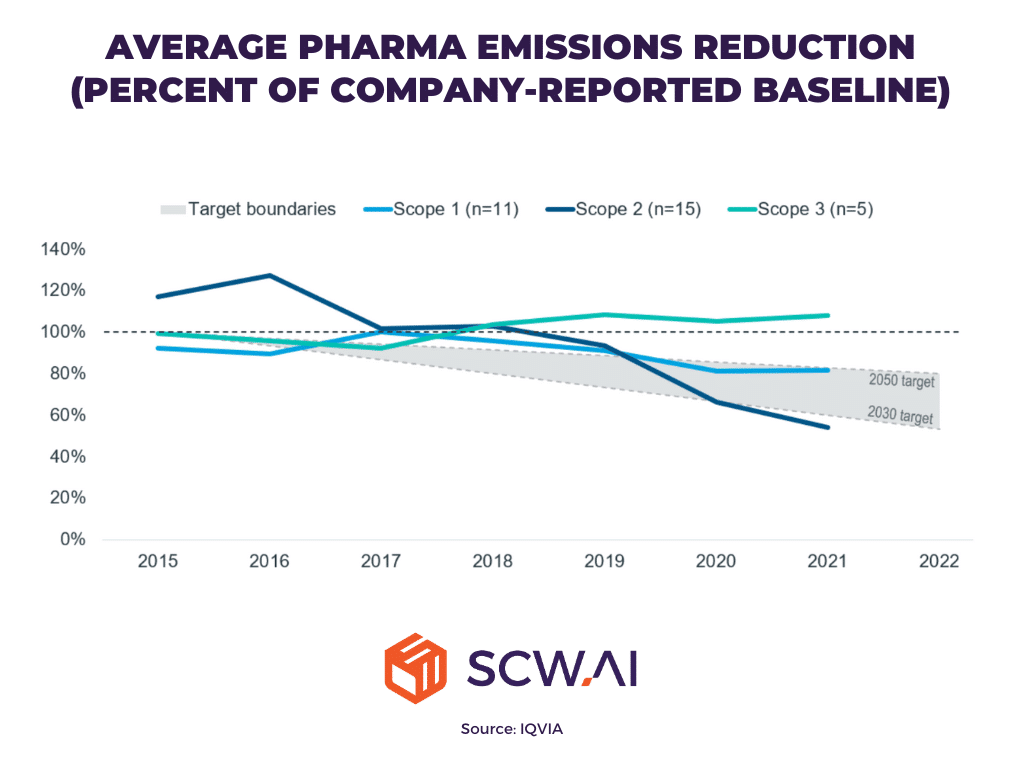
- Scope 1 Emissions: These are direct emissions from manufacturing activities. The pharma industry is on track to reach net zero for Scope 1 emissions by 2050, thanks to efficiency improvements and better monitoring capabilities as we will further discuss.
- Scope 2 Emissions: These relate to indirect emissions from the generation of purchased energy, such as electricity. Here, the industry is ahead of the curve, with a target of achieving net zero by 2030. The rapid reduction in Scope 2 emissions highlights a significant shift toward renewable energy sources like solar, wind, and bioenergy.
However, the real challenge lies with Scope 3 emissions, which account for approximately 80% of the pharmaceutical industry’s total greenhouse gas (GHG) emissions. Scope 3 emissions include indirect emissions from the entire supply chain—such as raw material extraction, transportation, and product disposal. Despite progress in other areas, there is currently no clear trend of reduction in Scope 3 emissions, indicating a critical gap in the industry’s sustainability efforts.
2. Water Stewardship in Pharma Manufacturing
The pharmaceutical industry is heavily dependent on water, with varying purity requirements for producing specific drugs. However, water scarcity is a growing global issue, with many densely populated regions across all continents facing the severe risk of water shortages. As concerns about water scarcity intensify, the pharmaceutical sector is under pressure to adopt more sustainable water management practices to ensure responsible usage without compromising product quality.
- Emerging Technologies for Water Recycling: Advanced water recycling technologies are at the forefront of this sustainability push. Techniques like reverse osmosis and membrane filtration are gaining traction due to their ability to purify and reuse wastewater effectively. Several case studies have shown that implementing these technologies can reduce water consumption by nearly 50% in some manufacturing facilities.
- Scrutiny of Water-Intensive Processes: A critical step towards better water management involves a thorough assessment of existing operations. Many pharmaceutical companies are conducting audits to identify water-intensive processes that can be optimized such as cleaning operations, cooling systems and solvent recovery.
3. Circular Economy
The concept of a circular economy centers around balancing the use of resources with the planet’s capacity to regenerate them. It involves creating systems that reduce, reuse, and recycle materials to minimize waste and decrease the dependency on finite resources. A stark reminder of the urgency for such models is the annual Earth Overshoot Day, which marks the date when humanity has consumed all the resources the Earth can regenerate in a year. As of 2024, we need 1.75 Earths to sustain current material usage —underscoring the need for circular economy practices across industries, including pharmaceuticals.

In recent years, the pharmaceutical sector has made some strides in improving circularity. Companies are exploring various strategies to minimize waste:
- Minimizing Waste with Digital Lean Practices: Many pharma companies are adopting lean manufacturing principles, supported by digital technologies, to reduce waste throughout production. For instance, if a pharma manufacturer can reach 100% first pass yield it can complete production in one batch without need for wasting extra virgin raw materials.
- Focus on Sustainable Packaging: According to a study by the European Federation of Pharmaceutical Industries and Associations, a majority of pharma companies identify packaging as a key area for enhancing circularity. Key initiatives include:
- Reducing single-use packaging:
- Innovative materials
- Developing eco-friendly packaging materials
- Implementing reverse logistics systems to facilitate the collection, sorting, and recycling of non-virgin packaging materials.
Key Challenges in Achieving Pharma Sustainability Goals
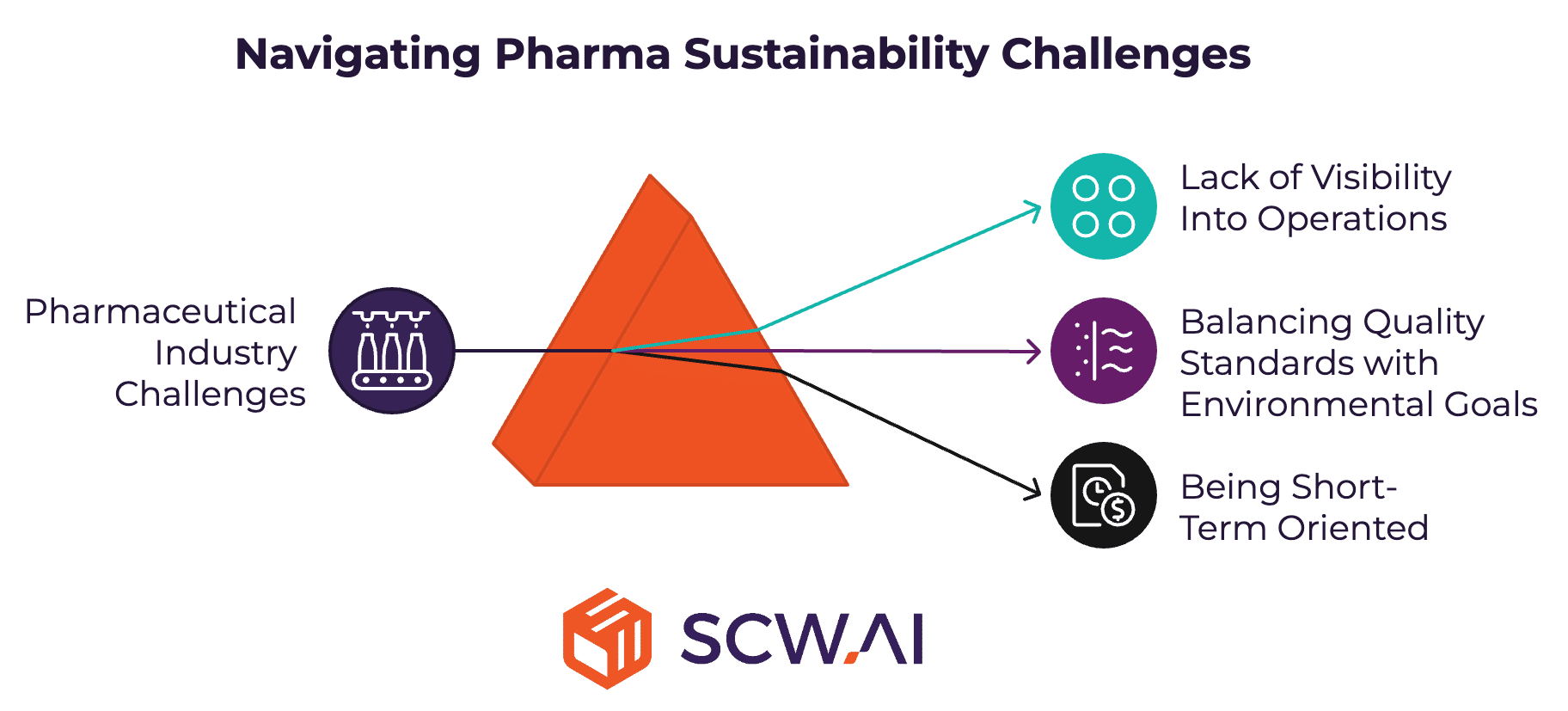
1. Lack of Visibility Into Operations
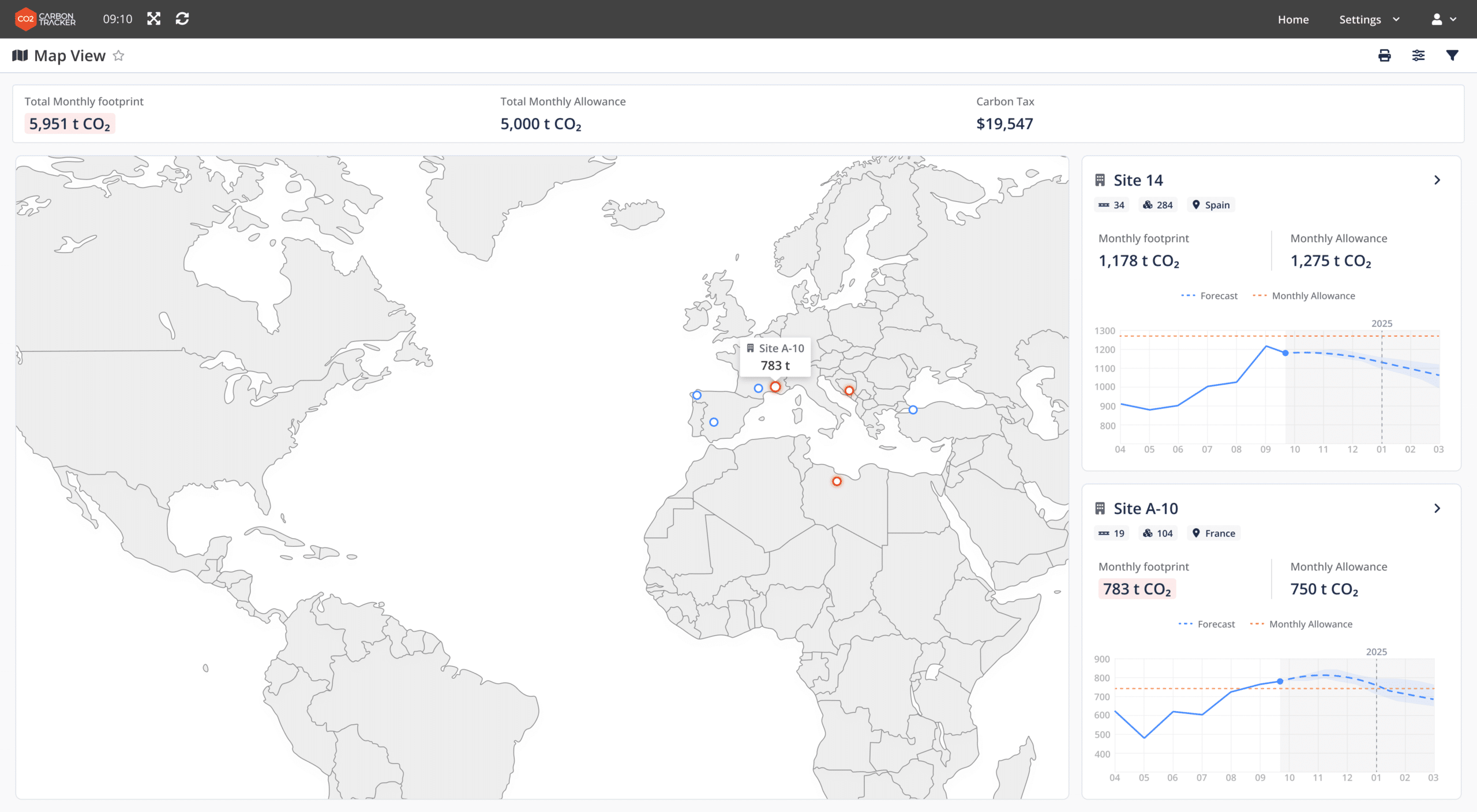
To improve business outcomes, pharmaceutical manufacturers must collect granular, accurate, real-time data and display it on digital interfaces (manufacturing analytics) to identify bottlenecks and monitor progress toward their goals. Without this capability, it becomes nearly impossible to determine the energy consumption of individual machines, measure inefficiencies in production, track Scope 3 emissions and many more—essential factors for sustainability in the pharmaceutical industry.
Achieving visibility, however, requires a robust digital infrastructure, which can be developed through investments in Pharma 4.0 technologies. According to Mike Walker, Executive Director of Global Healthcare and Life Sciences Digital Strategy at Microsoft, the pharmaceutical industry is still in the early stages of digital factory transformation, with only 2% to 3% of factories having achieved end-to-end visibility. Many pharmaceutical plants currently operate with disconnected data systems, making it challenging to gain a comprehensive view of their environmental impact especially for the supply chain activities.
2. Balancing Quality Standards with Environmental Goals
Meeting strict GMP regulations poses challenges for using sustainable packaging in the pharmaceutical industry. Packaging must ensure drug safety and stability, which makes recyclable or biodegradable options difficult to adopt. For example, blister packs, which mix plastic and aluminum, are effective but hard to recycle due to the difficulty of separating materials. Any new packaging also requires extensive testing and regulatory approval to confirm it does not compromise quality, slowing down the adoption of greener alternatives.
3. Being Short-Term Oriented
Today’s business mindset is frequently short-term focused, prioritizing outcomes for the next quarter or fiscal year. This short-term thinking often results in postponing or scaling back initiatives that could drive significant improvements in pharma sustainability. To make real progress, companies need to allocate dedicated budgets for sustainability projects, treating them as long-term investments rather than optional expenses.
Best Practices for Sustainable Pharma Manufacturing
1. Leveraging Advanced Technologies to Make Environmental Impact Visible
Following solutions can be useful for pharma companies to improve sustainability.
- Energy Trackers: These tools allow pharma manufacturers to monitor the energy usage of each production line in detail. By identifying inefficiencies and high-consumption areas, companies can take targeted actions to reduce energy waste and improve overall efficiency.
- Carbon Trackers: Carbon tracking solutions enable manufacturers to calculate their carbon footprint with precision, streamlining ESG (Environmental, Social, and Governance) reporting. These trackers can be further enhanced by digital twin technology, allowing manufacturers to simulate scenarios—such as increasing production or improving Overall Equipment Effectiveness (OEE)—to understand their potential impact on carbon emissions before making changes.
- AI Schedulers: Traditional scheduling often focuses on just-in-time delivery, changeover reduction, or profit maximization. AI-driven scheduling tools can now be programmed to prioritize minimizing the carbon footprint. These schedulers can optimize job shop operations to use energy more efficiently, such as scheduling energy-intensive tasks when solar power is at its peak.
- Data Gathering Solutions: The backbone of any sustainability strategy is accurate, real-time data. IoT devices, Modbus PLCs, and OPC connections are crucial for gathering detailed shop floor data, from energy usage to machine performance. This comprehensive data collection enables manufacturers to monitor, analyze, and optimize every aspect of their operations with sustainability in mind.
2. Minimizing Factory Waste
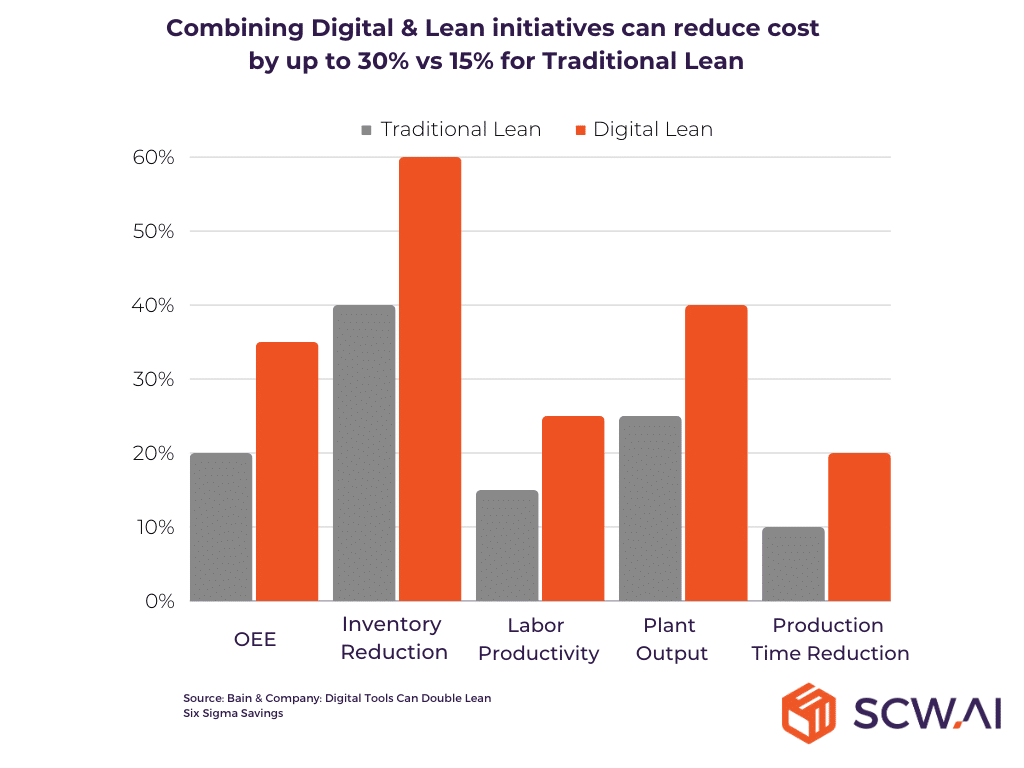
Implementing digital lean principles not only reduces costs and boosts throughput, but it also significantly lowers carbon emissions—improving the carbon footprint-to-revenue ratio. A compelling example comes from a World Economic Forum case study on Cipla’s Indian facility. Although Cipla’s primary goal was to enhance OEE and reduce downtime through digital Total Productive Maintenance and targeted changeover optimizations, these initiatives had a broader impact. Cipla achieved a 28% reduction in carbon emissions, alongside a 26% cost reduction and a 22% decrease in changeover times, showcasing how digital lean practices can simultaneously optimize processes, reduce waste, and eliminate inefficiencies.
The logic behind digital lean is straightforward. According to Bain & Company, digital lean initiatives can improve OEE by up to 35 percentage points. For example, if a pharmaceutical company’s initial OEE is 35%, an increase to 70% means they can produce the same output in just half the time. This efficiency allows for several sustainability benefits:
- Reduced Resource Utilization: With higher OEE, labor movements are minimized, and production time is shortened. This can mean closing the factory for half of the year, resulting in zero energy consumption during that period and reducing the facility’s overall carbon footprint.
- Lower Carbon Footprint per $1M Revenue: If a pharma company maintains production year-round with a higher OEE, its carbon footprint per million dollars of revenue decreases significantly. This is because the factory can produce double the output with almost the same energy and labor input, aside from raw materials.
3. Utilizing Paperless Quality Solutions
To comply with strict GMP regulations, the pharmaceutical industry generates thousands of log and batch entries to record every detail of shop floor activities. According to Indranil Nandi, Chief Scientific Officer at Jubilant, paper remains the primary medium for these reports, leading to piles of documents being filled and stored—even in today’s digital age.
Paper-based reporting is not only time-consuming and error-prone, increasing the chance of data entry mistakes by up to five times compared to digital systems. Additionally, the production of paper is resource-intensive and environmentally damaging, involving carbon-heavy processes, significant water consumption, and contributing to habitat destruction through deforestation. In fact, the paper industry causes deforestation each year on a scale comparable to the size of Pennsylvania or Greece. Paper waste further underscores its unsustainability for the pharma sector.
The alternative is GMP-compliant paperless quality solutions, such as Digital Logbooks and Digital Batch Records. These tools not only streamline operations but also adhere to ALCOA+ data integrity principles, reducing data entry errors and saving time. More importantly, they align with sustainable manufacturing practices by eliminating the environmental impact of paper use.

To explore how paperless solutions have transformed a major pharmaceutical manufacturer, download our case study and see the impact firsthand.
4. Enhancing Sustainable Supply Chains
Building a sustainable supply chain is a key component of achieving overall sustainability in the pharmaceutical industry. Many pharma companies are now adopting Supplier Codes of Conduct, which serve as a set of standards outlining the conditions suppliers must meet to collaborate with manufacturers. These codes typically address critical areas such as carbon footprint reduction, water conservation, ethical labor practices (including the prohibition of child labor), and waste management.
To further minimize environmental impact, the following strategies can be adopted:
- Local Sourcing: Whenever possible, pharmaceutical companies should sourcing raw materials and components from closer regions to reduce transportation-related greenhouse gas emissions.
- Electric Vehicles for Transportation: Utilizing electric vehicles (EVs) for transportation can reduce GHG emissions especially if factories have charging facilities in factories and factories have solar panels or source energy via a green producer.
- Sourcing Energy from Green Providers: Beyond transportation, companies are increasingly turning to renewable energy providers to power their operations. Thus, you can improve your sustainability while supporting green energy providers.
How SCW.AI Supports Pharma Sustainability
SCW.AI specializes in turnkey, end-to-end digital factory transformations for the pharmaceutical industry through our Digital Factory Platform. This platform gathers comprehensive data from the shop floor, providing decision-makers with critical insights via manufacturing analytics. These insights enable pharma manufacturers to make more informed, data-driven decisions that enhance both operational efficiency and sustainability.
Our platform also includes AI-driven capabilities that automate and streamline key processes, such as anomaly detection and optimized scheduling. In addition, SCW.AI offers GMP-compliant Digital Logbooks, which replace traditional paper-based documentation. With our solutions, pharmaceutical companies can closely monitor their environmental impact, reduce production waste and paper waste.
To learn more about pharma sustainability and how SCW.AI can help, feel free to contact us.
If you want to see our Digital Factory Platform in action, book a demo today.