Europe’s medicine shortage crisis has reached a point where incremental fixes are no longer sufficient. Persistent shortages of antibiotics, oncology medicines, cardiovascular treatments, and other essential therapies are now recognized as a structural risk to public health and healthcare systems across Europe.
The Critical Medicines Act (CMA) represents the European Commission’s most comprehensive response to date. Yet legislation alone cannot restore supply security. The decisive factor will be whether Europe succeeds in rebuilding competitive manufacturing capacity—quickly, sustainably, and at scale.
This article summarizes the key findings of a report developed in collaboration with Medicines for Europe and SCW.AI. It demonstrates why Factory Modernization is the most effective and realistic path to strengthening Europe’s pharmaceutical manufacturing base under the CMA.

The full report (PDF) provide deeper analysis, quantified models, and real-world case studies. By clicking the button below and filling out the form, you can download the full report for free!
1. Europe’s Medicine Shortage Crisis

Medicine shortages are no longer isolated incidents caused by temporary disruptions. Across Europe, shortages have become systemic and recurrent, affecting nearly all therapeutic areas and healthcare settings.


What distinguishes the current crisis from previous episodes is its structural nature. Supply fragility is now embedded in the economic, and industrial foundations of pharmaceutical manufacturing in Europe.
Manufacturing Failures as the Primary Root Cause
Nearly 50% of all medicine shortages in Europe originate directly from manufacturing failures, including:
- ~30% stem from capacity constraints,
- ~15% from slower-than-target production speeds, and
- ~5% from unavailability of APIs.
This reality reframes the problem: medicine availability is fundamentally a manufacturing productivity and resilience issue.

2. Structural Issues in Europe’s Pharmaceutical Supply Base
Several long-term trends have gradually eroded Europe’s manufacturing resilience and increase possibility of drug shortages.
Supplier Concentration and Dependency
46% of critical generic medicines rely on a single supplier
Supply disruptions at one site or supplier can immediately translate into continent-wide shortages as reported per IQVIA.

API Dependency and Loss of Strategic Autonomy
European manufacturers now hold only ~one third of Certificates of Suitability (CEPs)
This figure stood at ~59% in 2000, highlighting a two-decade erosion of domestic capability
The result is a supply chain heavily dependent on non-EU (especially Asia) regions for both APIs and finished dosage forms.

3. Economic & Demographic Pressures
Off-patent medicines (generics and biosimilars) constitute 90% of critical medicines by volume in Europe. They also account for 70% of prescribed treatments. Yet, the manufacturers supplying these products operate under economic pressure.
Price Compression and Procurement Practices
- Lowest-price procurement dominates many EU markets
- Margins have steadily declined despite rising input costs
- Supply reliability and resilience are often not rewarded economically

Capital Trapped in Compliance
European off-patent manufacturers now allocate approximately 70% of capital investment to:
- Sustainability compliance
- Asset depreciation
- Mandatory things to keep the lights on.
While essential, these investments leave little room for productivity-enhancing modernization, trapping manufacturers in a low-productivity, low-resilience cycle.
Labor Constraints: The Hidden Capacity Bottleneck
Europe’s demographic and labor market realities further limit traditional capacity expansion.
Machine and plant operators are now the second most severe labor shortage occupation in the EU
An aging workforce and declining technical labor pool exacerbate the challenge
In this context, increasing production by simply adding more people or shifts is increasingly unrealistic. Europe must increase pharmaceutical output with fewer operators and higher skill leverage.
4. The Critical Medicines Act: Creating the Right Policy Conditions
The Critical Medicines Act acknowledges that medicine supply security is a strategic priority requiring industrial policy intervention.
Key elements include:
MEAT procurement criteria, rewarding resilience, sustainability, and quality
Strategic Projects to support domestic capacity and reduce dependency including factory modernization
Financial and regulatory mechanisms to lower investment risk
These measures are essential for pharmaceutical sovereignty of Europe, but policy alone cannot create capacity. Capacity is created on factory floors.

5. Why Factory Modernization Is the Most Effective Domestic Capacity Building Strategy
There are only two ways to increase pharmaceutical production:
Build new factories and hire more workers
Increase output from existing assets through productivity gains
Given Europe’s labor shortages, capital constraints, and long permitting timelines, the second option is both faster and more scalable.
On the other hand, factory modernization supports data-driven decision making, responsiveness in real-time and intelligent automation which are crucial for scientific shop floor management thus it creates a positive feedback loop for various manufacturing KPIs that are the real improvements in productivity.

The Productivity Gap in Pharmaceutical Manufacturing
Benchmarking overall equipment effectiveness (OEE) across pharmaceutical plants reveals a striking performance gap:
Typical EU factories operate at ~37% OEE
Digitally modern factories reach ~60% OEE
This gap represents latent capacity already embedded in existing infrastructure.


Quantified Impact of Closing the Gap
Moving from 37% to 60% OEE enables:
~30% increase in throughput
~24% reduction in operating costs
Improved OTIF, quality consistency, and compliance
In effect, digital modernization delivers capacity equivalent to building new production lines—without new bricks, machines, or headcount.

6. Pharmaceutical Factory Modernization
Today, only an estimated 3–5% of European pharmaceutical manufacturing sites can be considered fully modernized, with integrated data foundations, real-time visibility, and advanced optimization capabilities. The vast majority of factories still operate with fragmented systems, manual processes, and paper-based workflows.
Factory Modernization is therefore not a single technology leap, but a structured progression. Each layer builds on the previous one, collectively enabling productivity, compliance, resilience, and—eventually—AI-driven self-driving shop floors.
Data Foundation: Building the Backbone of Pharma Factory Modernization
A robust data foundation is the non-negotiable starting point of any modernization journey. Without it, all higher-level digital initiatives remain fragile or ineffective.
At its core, the data foundation connects the physical factory to digital systems through:
PLC and OPC integrations
IoT devices and sensors, RFID Cards
Automated, time-stamped, high-frequency data capture directly from equipment and processes
This real-time, granular data backbone enables:
Manufacturing, labor, asset, and sustainability analytics
Lean management dashboards and daily performance monitoring
Algorithm-driven production scheduling
Paperless quality systems and digital batch records
All forms of advanced analytics and AI models

Monitoring & Visibility: Maximizing Line, Labor, and Asset Performance
Modern pharmaceutical factories use real-time analytics to:
Track manufacturing KPIs
Understand labor utilization and skill deployment
Identify bottlenecks, chronic losses, and recurring deviations
Link downtime, changeovers, and quality deviations to root causes
For managers, this eliminates reliance on retrospective reports and manual investigations. Decisions shift from reactive to proactive, enabling faster interventions, better prioritization, and continuous improvement across lines, shifts, and sites.
Daily Performance Boards and Digital Work Instructions for Lean Execution
Visibility alone does not guarantee execution. The next step is embedding insight into daily routines.
Digital SQCDP boards replace static whiteboards by presenting real-time performance across safety, quality, cost, delivery, and people. Deviations are automatically flagged, enabling focused daily management meetings and clear accountability.
Digital work instructions ensure standardized execution of complex tasks such as:
Changeovers
Quality checks
Maintenance activities
By guiding operators step-by-step with visual, interactive instructions, factories improve consistency, reduce errors, and significantly accelerate onboarding—an important advantage given Europe’s labor shortages.
Paperless GMP Reporting: Streamlining Compliance and Audit Readiness
On average, 10% of staffed time is spent on manual paperwork, and in some operations this can be substantially higher. Paperless quality solutions can:
Speed up reporting 5–10×
Reduce data entry errors by up to 85%
Enforce completeness and data integrity by design
For large pharmaceutical factories, this also addresses a significant sustainability issue. Many sites consume hundreds of thousands—or even millions—of paper pages annually for GMP reporting. The global paper industry contributes to deforestation on a scale exceeding the land area of Portugal each year, meaning pharmaceutical manufacturing inadvertently adds to environmental degradation through manual documentation alone.
Paperless quality systems therefore deliver simultaneous gains in productivity, compliance, audit readiness, and environmental performance.

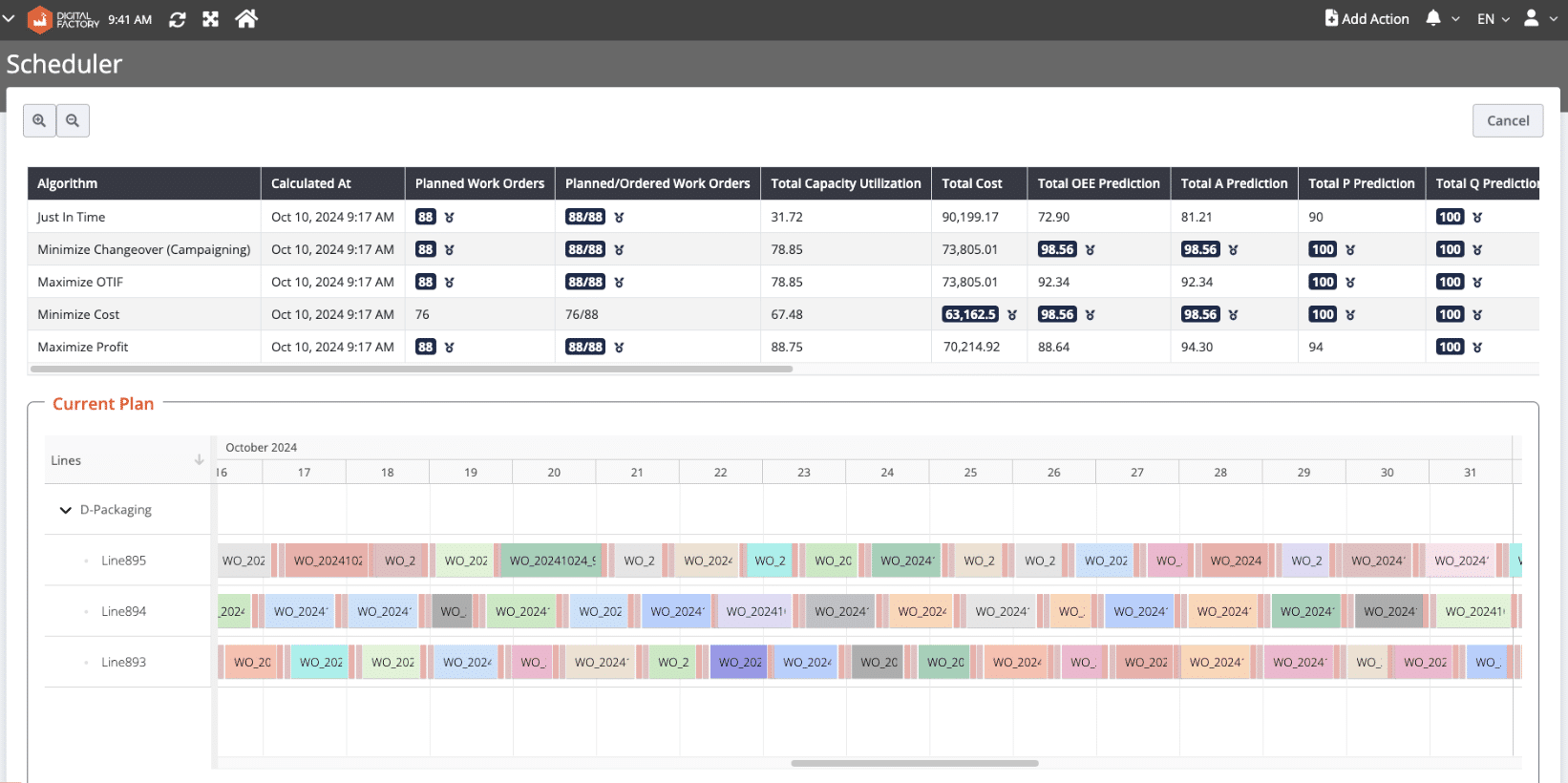
Responsive Production Scheduling: for Maximum Productivity
Pharmaceutical production scheduling is inherently complex, constrained by quality requirements, cleaning rules, labor availability, and demand volatility. Modern scheduling tools address this complexity through:
Real-time schedule adherence and OTIF monitoring
Visual Gantt-based planning for lines, labor, and assets
Scenario testing to balance changeovers, costs, service levels, and capacity
By replacing static spreadsheets with algorithm-supported scheduling, factories improve responsiveness to disruptions, reduce idle time, and increase effective capacity—directly strengthening supply reliability for critical medicines.
AI in Pharma Manufacturing: Outsmarting the Competition—When the Factory Is Ready
Artificial intelligence offers significant upside for pharmaceutical manufacturing. According to PwC, AI-enabled shop floor use cases could generate over $10 billion in value for pharmaceutical factories in the EEA and U.K. alone.

However, AI effectiveness depends entirely on digital maturity. Reliable AI decisions require:
High-quality, real-time data
Clear data contextualization through analytics and dashboards
Digitized scheduling, execution, and quality processes
Today, around 95% of EU pharmaceutical manufacturers must first reach this level of readiness before AI can deliver compliant, trustworthy results.
Once readiness is achieved, five AI use cases stand out as particularly high-impact:
Predictive maintenance, reducing unplanned downtime
Scenario-based AI scheduling, optimizing cost, service, and throughput
Computer vision–based quality inspection, reducing manual checks
Automated task assignment, improving execution speed and consistency
In this sequence, AI becomes not a leap of faith, but the logical extension of a digitally modern factory.

Proof from the World Economic Forum Lighthouse Network
The World Economic Forum Lighthouse Network provides independent validation of digital factory transformation.
189 Lighthouse factories recognized globally
23 in pharmaceuticals and medical devices
Over 60% located in South and East Asia
Less than 25% in the EU
This geographic imbalance signals a competitive risk: global peers are modernizing faster than Europe.

Pharmaceutical Lighthouse Results
Across validated case studies, manufacturers achieved:
Up to 37 percentage point OEE improvements
up to 90% reduction in unplanned downtime
–20% to –30% operating cost reductions
Significant quality and sustainability gains
These outcomes are audited, repeatable, and achieved under GMP conditions.


7. CMA Policy Suggestions
For the Critical Medicines Act to achieve its objectives, modernization must be defined holistically.
Strategic Projects should support:
Digitally mature factories ready for AI deployment
95% of factories that must first build digital foundations to effectively harness AI.
Modernization should be understood as investments that:
Increase capital and labor productivity
Enhance resilience, agility, and quality
Expand effective production capacity
—without necessarily adding new physical assets or workforce
This approach ensures that public funds generate measurable, long-term productivity gains.

Why Accessing the Full Report
This article presents a summary. The full PDF report provides:
Detailed quantitative modeling
Factory-level productivity and cost scenarios
Detailed use cases of various factory modernization technologies
Complete analysis of AI readiness of EU pharma producers and roadmap for deploying 5 different AI applications for manufacturers
In-depth Lighthouse case studies
Comprehensive policy advises
- Data driven economic and demographic analysis of European pharma industry
Complimentary Free Webinar on CMA & Factory Modernization

The online webinar, taking place on February 18th at 04:00 PM GST, offers the opportunity to engage directly with Adrian van den Hoven, Director General of Medicines for Europe, and Evren Ozkaya, PhD, founder and CEO of SCW.AI. These experts will explore the implications of full PDF report for both policy and industry.
Get Your Free eBook Now!
Fill out the form below to receive a free copy of the full report, “Securing Europe’s Medicine Supply: Can Factory Modernization Deliver Supply Resilience Under the Critical Medicines Act?“.