Regulatory bodies like the FDA strive to enhance the quality of pharmaceutical products, ultimately aiming to improve public well-being. The quality management maturity initiative is a significant step in this direction. At SCW.AI, we believe the QMM program offers substantial benefits for U.S. pharmaceutical manufacturers who prioritize quality beyond the minimum compliance with cGMP standards and invest in the future of pharmaceutical manufacturing to achieve world class manufacturing excellence.
In this article, we aim to provide an in-depth exploration of quality management maturity from the perspective of its relevance to pharmaceutical manufacturers. We will begin by defining the concept and its primary objectives. Following this, we will delve into its benefits for pharma manufacturers and examine the synergy between Pharma 4.0 solutions and quality excellence in the industry. Lastly, we will address frequently asked questions about quality management maturity and outline how SCW.AI can support pharma manufacturers in enhancing quality and resilience.

What is Quality Management Maturity?
Quality Management Maturity (QMM) is a structured, qualitative approach to assessing the quality commitment and operational resilience of pharmaceutical manufacturers. Championed by the FDA’s Center for Drug Evaluation and Research (CDER), the QMM initiative aligns with regulatory objectives to elevate industry standards, ensuring that manufacturers not only comply with cGMP regulations but also strive for continuous improvement.
QMM evaluates critical aspects of a manufacturer’s operations, including:
- Quality Policies: The robustness and effectiveness of quality management practices.
- Resilience: The ability of manufacturing and supply chains to withstand disruptions and ensure business continuity.
- Technological Infrastructure: The adoption and utilization of advanced technologies to enhance quality, agility, and sustainability in pharmaceutical operations.
What is the Main Purpose of QMM?
The primary purpose of QMM is to promote excellence in pharmaceutical manufacturing by distinguishing companies that invest in superior quality and operational resilience. In doing so, FDA aims to address systemic drug shortages in the U.S. Through the QMM assessment, the FDA provides market signals to stakeholders, including group purchasing organizations (GPOs), highlighting manufacturers that prioritize quality and agility. This encourages a shift away from procurement decisions driven solely by cost.
In 2020, the FDA identified the lack of market signals to differentiate world-class pharmaceutical manufacturers as a contributing factor to drug shortages. Currently, adherence to cGMP is the only metric available for GPOs to assess suppliers, making price the dominant selection criterion. With four major GPOs controlling 90% of the U.S. medicine procurement market, manufacturers often cease production of older, low-profit drugs due to their limited bargaining power, causing shortages. The QMM framework offers a qualitative evaluation of quality maturity, enabling GPOs to prioritize manufacturers committed to innovation, resilience, and sustainability alongside GMP compliance and price considerations.
The 5 Pillars of QMM Assessment
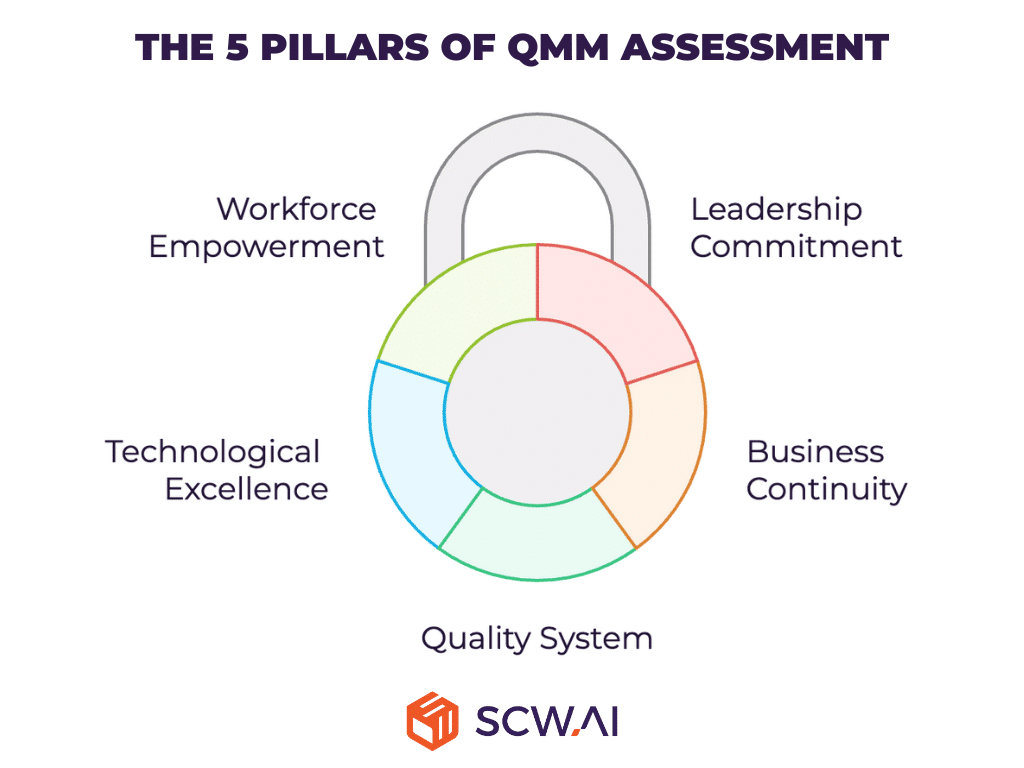
1. Leadership Commitment: Driving Quality from the Top Down
Strong leadership is the cornerstone of any quality-focused organization. QMM assesses the degree to which leadership fosters a culture of quality, ensuring it is embedded across all levels of the company. This includes strategic decision-making, resource allocation, and consistent communication of quality goals. Leadership that prioritizes quality not only drives operational excellence but also inspires accountability and continuous improvement throughout the organization.
2. Ensuring Business Continuity
Resilience in manufacturing and supply chain operations is vital to maintaining an uninterrupted supply of medicines. QMM evaluates a company’s preparedness to handle disruptions, such as supply chain bottlenecks or latency in production due to unplanned downtime. Manufacturers with robust business continuity plans rich greater OTIF scores and create sustained relationships with customers.
3. Advanced Pharmaceutical Quality System
A mature pharmaceutical quality system (PQS) goes beyond compliance with cGMP standards. QMM assesses how effectively companies integrate proactive quality management practices, including real-time monitoring, risk-based decision-making, and corrective actions.
4. Harnessing the Power of Technological Excellence
The integration of advanced technologies is a critical driver of operational agility and product quality. QMM evaluates how manufacturers utilize tools such as GMP compliant logbooks, artificial intelligence (AI/ML models), and manufacturing analytics to reduce variability, and enhance efficiency.
5. Empowering and Upskilling a Future-Ready Workforce
People are the backbone of any quality initiative. QMM assesses a manufacturer’s investment in workforce development, focusing on training, upskilling, and employee engagement. This way initiatives for total productive maintenance and lean manufacturing provides higher return while processes are standardized thanks to common corporate knowledge base.
Top 5 Benefits of QMM for Pharma Manufacturers
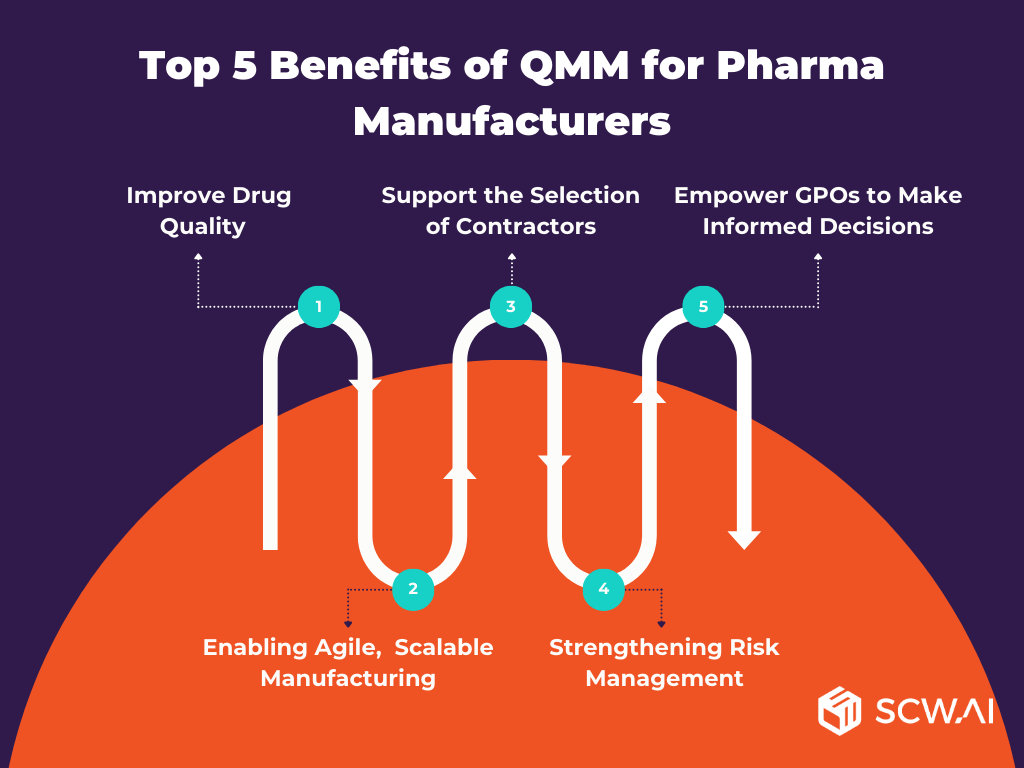
1. Improve Drug Quality and Ensure Compliance
By fostering a proactive approach to quality management, QMM helps manufacturers consistently produce high-quality drugs that meet regulatory requirements and even more. It encourages continuous improvement and alignment with evolving industry expectations.
2. Enabling Agile, Efficient, and Scalable Manufacturing
QMM promotes operational flexibility by integrating advanced technologies and best practices. Manufacturers can quickly adapt to market changes, scale production efficiently, and reduce waste. This agility ensures a faster response to demand surges, such as those experienced during Covid-19 pandemic. Thus, people can obtain medicines in any condition as discussed in the video below.
3. Support the Selection of Quality-Focused Contract Manufacturers
Pharmaceutical companies can use QMM assessments to identify partners who share their commitment to excellence, reducing risks in outsourcing and ensuring consistent product quality across the supply chain.
4. Strengthening Risk Management Across Manufacturing Operations
QMM emphasizes a structured approach to identifying, evaluating, and mitigating risks throughout the manufacturing process. By leveraging real-time data, manufacturers can address potential disruptions, such as equipment failures or supply chain bottlenecks. Enhanced risk management reduces downtime, minimizes financial losses, and ensures continuity in drug production and supply.
5. Empower Bulk Medicine Purchasers to Make Informed Decisions
With QMM assessments providing transparency into manufacturers’ quality practices, bulk medicine purchasers, such as GPOs, can make more informed procurement decisions. This shifts the focus from cost alone to a holistic evaluation of quality and resilience, rewarding manufacturers who prioritize long-term value.
Pharma 4.0 Solutions: Unlocking Pharma Manufacturing Excellence
By leveraging pharma 4.0 solutions below, companies can modernize their operations and align with the goals of the QMM framework.
1. Real-Time Monitoring Solutions: Stay Ahead of Quality Issues
Monitoring tools, such as OEE Tracker, enable manufacturers to oversee critical manufacturing KPIs in real-time. For instance by analyzing components of overall equipment effectiveness (OEE) like availability, performance, and quality percentages, manufacturers can identify bottlenecks. By further measuring adherence to OEE targets, manufacturers can pinpoint areas for improvement, track the effectiveness of processes, and drive continuous improvement initiatives.
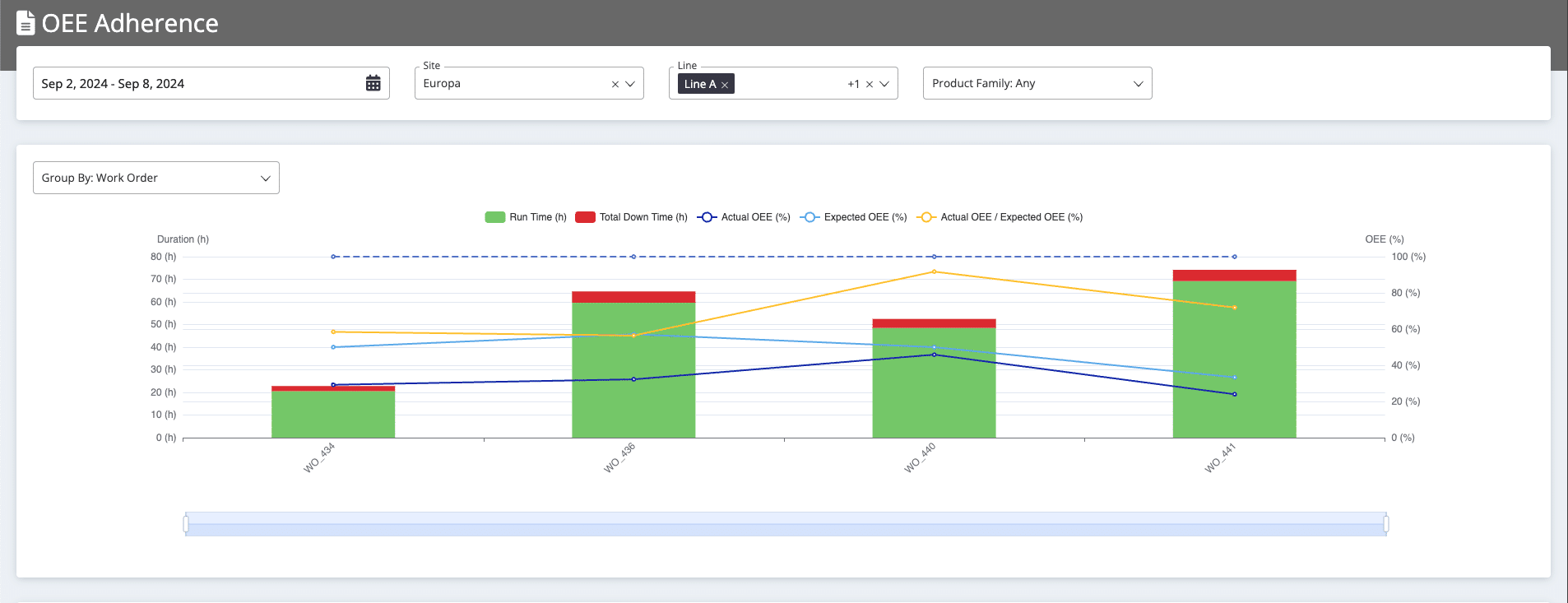
In addition, keeping an eye on metrics such as first pass yield allow granular tracking of scrap rates and rework requirements. By isolating specific equipment causing non-ideal quality, immediate corrective actions can be taken to prevent further disruptions.
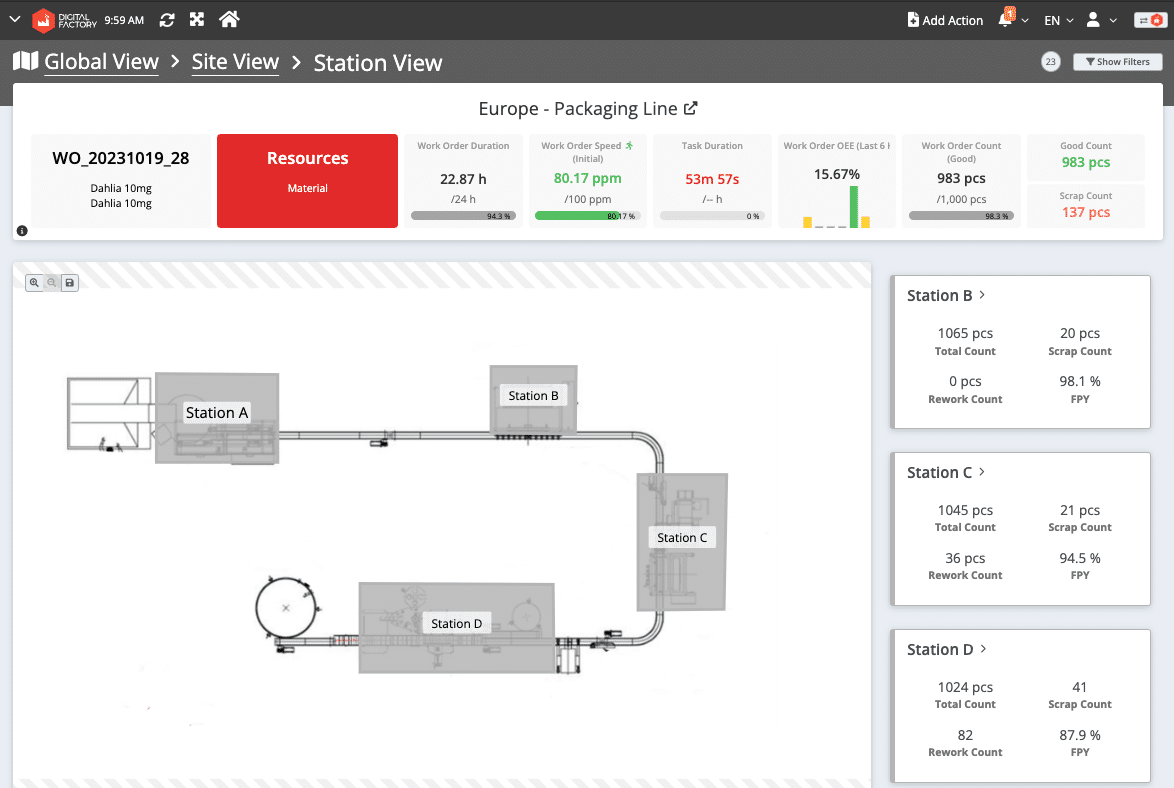
2. Seamless Execution Solutions for Greater Efficiency
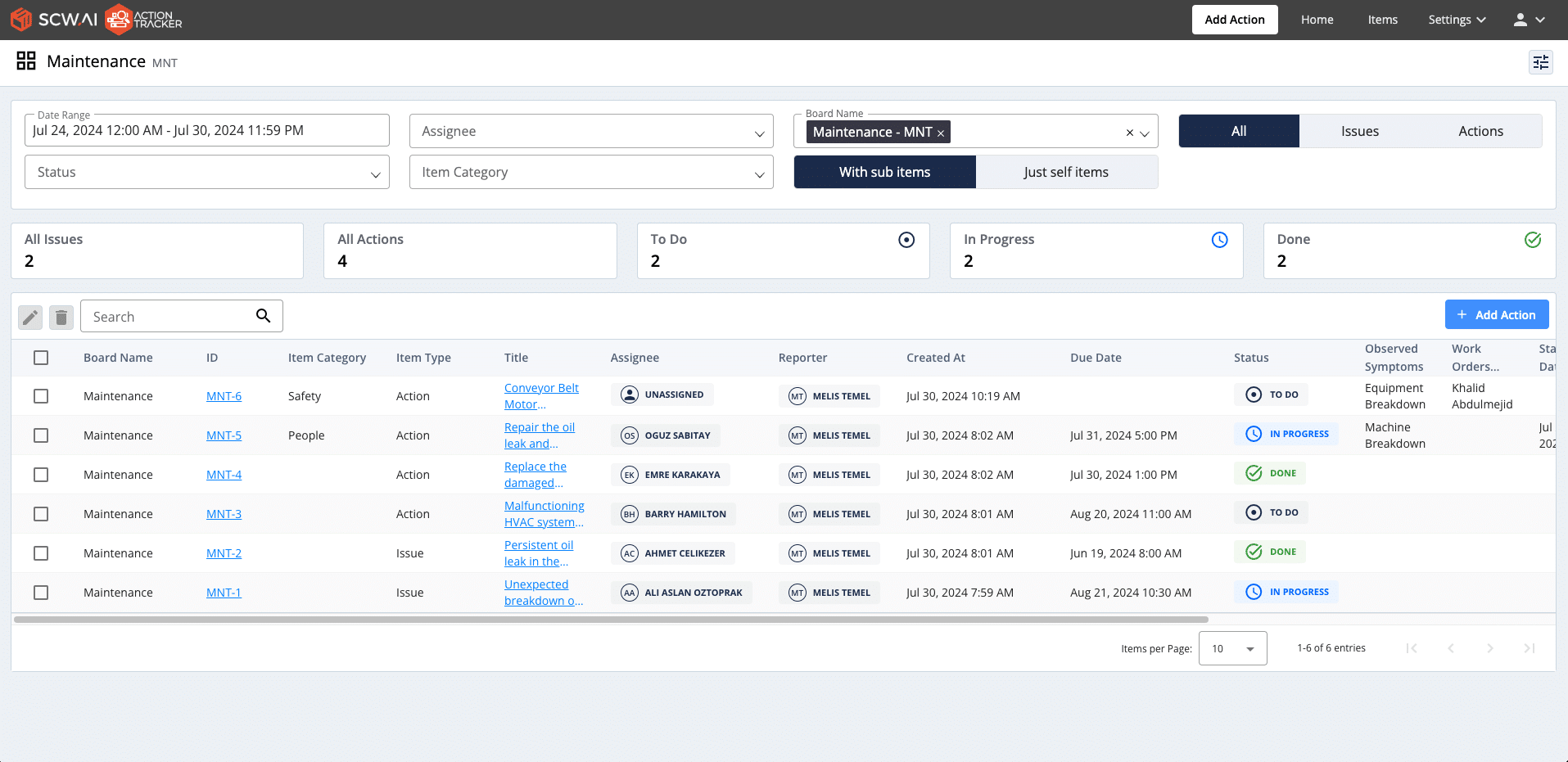
Manufacturing execution tools, like Action Tracker, streamline issue management by providing a user-friendly digital interface to define problems, assign responsibilities, and set due dates for resolution. Thus, manufacturers can execute tasks more effectively, minimizing downtime and maximizing productivity. This approach improves:
- Accountability: Managers can monitor task status in real-time, ensuring timely completion.
- Efficiency: Defined workflows enhance team collaboration and reduce operational delays.
3. Paperless Quality Solutions for a Future-Proof Process
GMP-compliant paperless manufacturing tools, such as Digital Logbook and Batch Record, help manufacturers adhere to regulatory standards like FDA 21 CFR Part 11 or Eudralex Volume 4 by design. These tools deliver:
- Enhanced Compliance: Streamlined reporting processes reduce time and errors for data entry by up to 85%, as demonstrated by case studies.
- Standardized Operations: Digital SOPs embedded in the system ensure tasks are executed consistently.
- Proactive Quality Management: Alerts for out-of-specification deviations enable timely corrective actions, preventing quality issues before they escalate.

4. Smarter Planning Solutions for Resilient Operations
Advanced planning and scheduling tools elevate manufacturers’ agility and operational quality through innovative technologies.
For instance, AI Scheduling algorithms generate optimal schedules based on objectives like minimizing changeovers, maximizing OTIF, ensuring JIT production and many more. Simulations allow planners to choose schedules that best align with business goals, potentially reducing costs by up to 20% in certain scenarios and supporting business continuity.
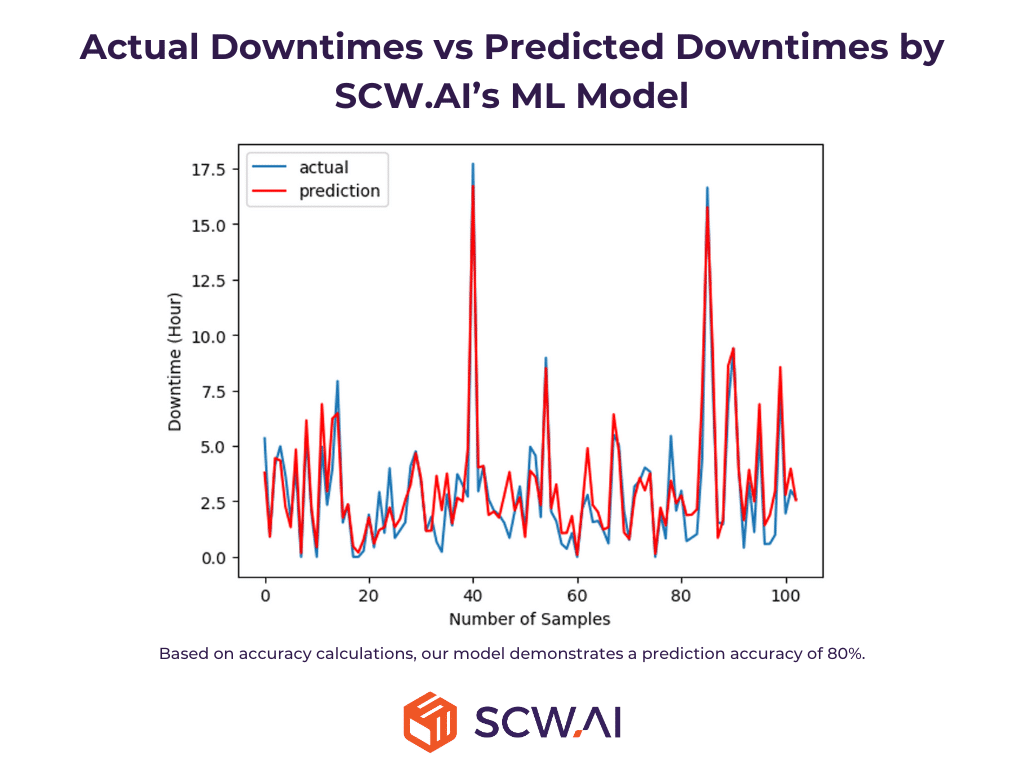
5. AI/ML Solutions for Intelligent Automation
Machine learning driven predictive maintenance models identify signs of equipment failure or performance degradation, enabling planned maintenance before downtime or quality issues arise. In addition, computer vision models can intelligently automate quality-check processes by examining the outputs of production lines. Moreover, anomaly detection algorithms can analyze historical machine configuration data and notify executives of deviations from typical machine setups.
Thus, manufacturing AI ensures operational continuity while preserving product quality and reducing costs.
Navigate the world of AI in pharma with our free, accessible white paper. Discover key statistics, future expectations, real-world applications, and inspiring case studies. Download your copy today!
Maximize Quality and Resilience with SCW.AI
Our Digital Factory Platform is tailored to meet the specific digitalization needs of pharmaceutical manufacturers and can be deployed with ease and speed. As a modular solution, it is ideal for factories just beginning their digital transformation journey as well as those looking to enhance their digital infrastructure with additional tools.
We empower pharmaceutical manufacturers with solutions for:
- Monitoring
- Execution
- Paperless Quality
- Planning
- AI-driven insights
To learn more about how our solutions can elevate your Quality Management Maturity (QMM), feel free to contact us.
Experience the transformative potential of the Digital Factory Platform—book a demo now.
QMM FAQs
1. Does QMM Assassement Effect GMP Compliance?
No, QMM is independent of cGMP compliance. While cGMP sets the baseline regulatory requirements for pharmaceutical manufacturing, QMM focuses on assessing a manufacturer’s quality management maturity and operational excellence beyond basic compliance.
2. Does QMM Assassement Evaluate Product Quality
No, QMM assessments do not evaluate product quality. The FDA conducts product quality evaluations regularly using various methods. The purpose of QMM is to assess the manufacturing environment and managerial approach that support product quality, rather than the quality of the products themselves.
3. Does QMM Evaluation Include Product Quality Metrics?
No, QMM is not a quantitative assessment. However, pharmaceutical companies committed to continuous improvement and world-class quality often utilize a range of metrics, such as: OEE, first pass yield, planned maintenance percentage etc. These metrics and many more help manufacturers monitor and enhance their processes. If you are interested in exploring manufacturing KPIs, you can download our Manufacturing KPIs Handbook for Managers and Executives today.