In pharmaceutical manufacturing, a single misstep can compromise product quality, trigger regulatory penalties, and cause major financial losses. One critical factor often overlooked is process hold time—the validated window during which materials, intermediates, or bulk products can remain at a given stage without compromising quality, safety, or efficacy.
Traditionally, managing hold time has relied on manual checks and paper-based records. In the digital era of Pharma 4.0, these methods are no longer sustainable. Poor hold time management not only risks product degradation but also creates costly inefficiencies. Equipment and skilled staff may sit idle when production steps are out of sync, driving up waste and reducing profitability.
This article explores how modern, automated approaches can transform hold time management from a compliance burden into a strategic lever—helping manufacturers stay audit-ready, minimize waste, and achieve world class manufacturing.
Understanding Process Hold Time
Process hold time is a cornerstone of pharmaceutical quality assurance, defined and enforced by regulatory bodies such as the WHO, FDA, and EMA. It specifies the maximum duration materials, intermediates, or bulk products can remain at a processing stage without compromising quality, safety, or efficacy. By establishing scientifically validated limits, manufacturers mitigate risks such as degradation, microbial growth, or chemical instability that could render a batch unusable.
However, in practice, hold time in pharmaceutical manufacturing extends beyond this regulatory definition. It can serve multiple purposes:
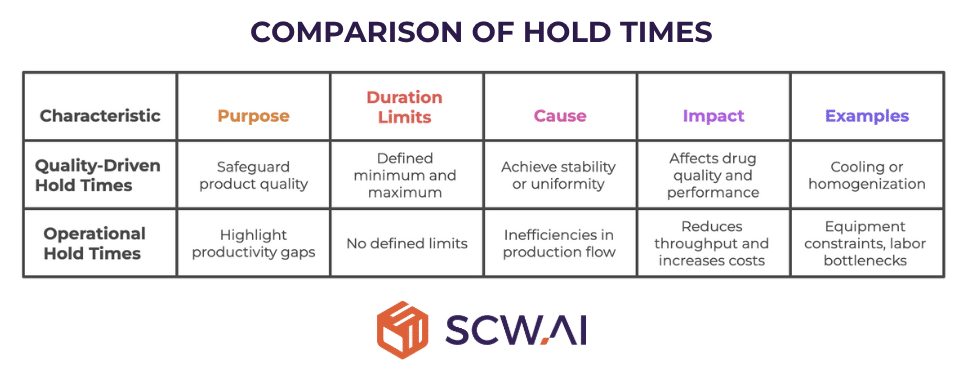
Quality-driven Hold Times
In some processes, materials must rest for a defined duration to achieve stability or uniformity. For example before granulation, manufacturers may require a product to rest for a minimum of 1 hour and a maximum of 3 hours to ensure proper cooling or homogenization. In such cases, adherence to both the minimum and maximum limits directly affects drug quality and therapeutic performance.
To manage the production of medicines such as biologics, ATMPs, radiopharmaceuticals it is crucial to prepare effective just-in-time schedules that respect quality-driven hold times. In addition, precise execution of these schedules becomes vital via high levels of factory visibility to ensure reliability of these sensitive pharmaceuticals.
Operational Hold Times
In other situations, no quality-driven minimum or maximum duration exists. Instead, materials are held due to inefficiencies in production flow. These idle times do not contribute to quality assurance but highlight productivity gaps on the shop floor, such as:
- Equipment Constraints: An intermediate product cannot advance because the line is still busy with a previous batch, often linked to lower OEE (Overall Equipment Effectiveness) or unexpected cycle time increases.
- Labor Bottlenecks: Operators require extra time to set up the next line or perform changeovers, delaying the transfer of materials.
- Scheduling Misalignment: Poor job shop scheduling or low schedule adherence causes materials to sit idle unnecessarily, creating non–value-added hold times.
In short, process hold time can be either intentional or unintentional. When scientifically justified, it safeguards product quality and must be monitored to ensure safe and effective therapies. When caused by inefficiencies, it reduces throughput, drives up costs, and undermines overall production capacity. Recognizing and managing both sides of hold time is essential for balancing compliance, efficiency, and profitability.
The Science of Quality Related Hold Time: Validation and Study
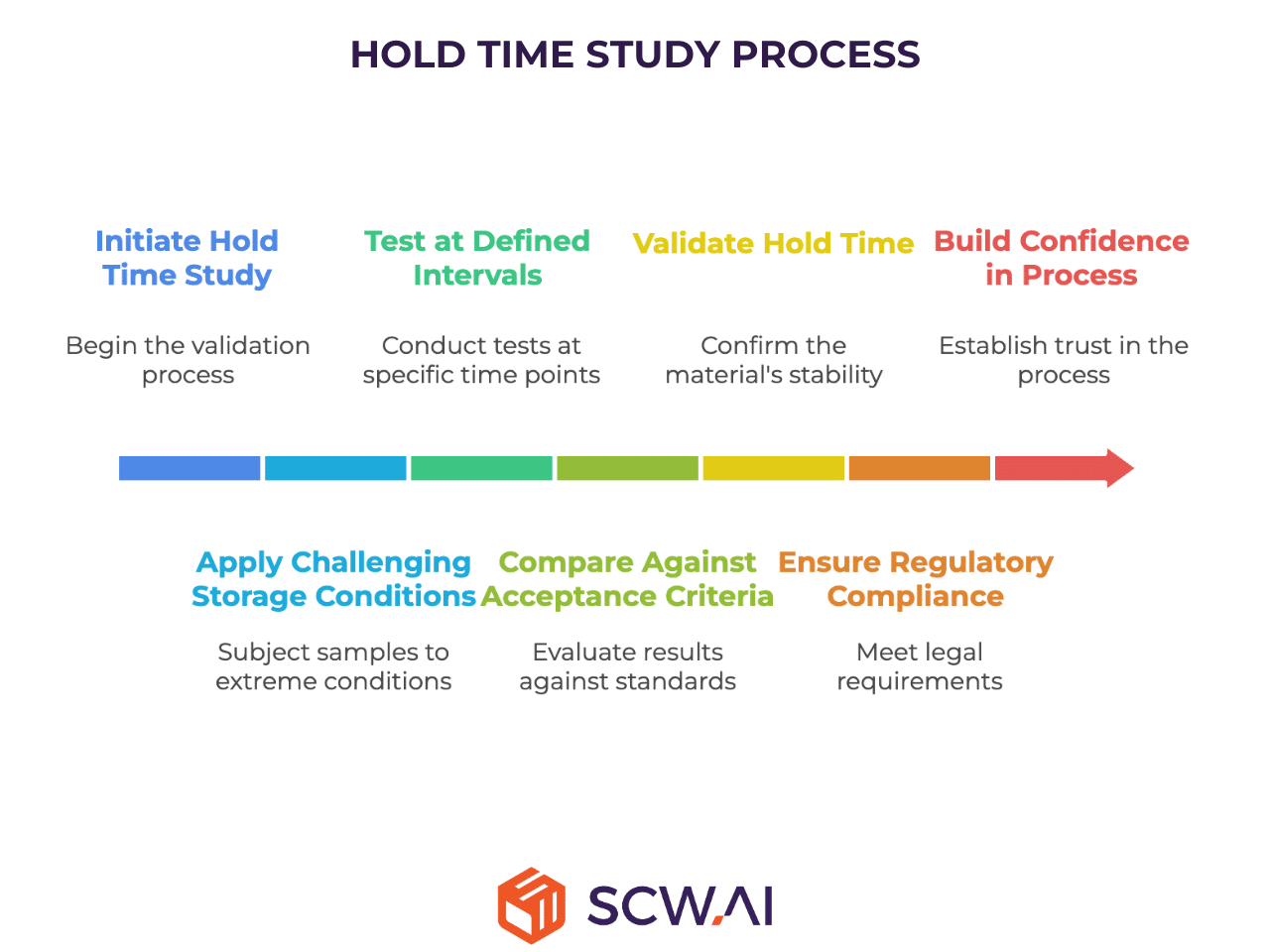
Quality related hold time is never arbitrary. Before it becomes part of a process, it must be scientifically validated through a hold time study. This validation provides documented evidence that a material remains within predefined specifications over the approved duration.
Key elements of a hold time study include:
- Challenging storage conditions applied to representative samples.
- Testing at defined intervals for attributes such as assay, impurity levels, moisture content, and microbial load.
- Comparisons against acceptance criteria to confirm product integrity.
Successful validation not only supports regulatory compliance but also builds confidence in the reliability of the process.
Calculation of Hold Time in Pharma Manufacturing
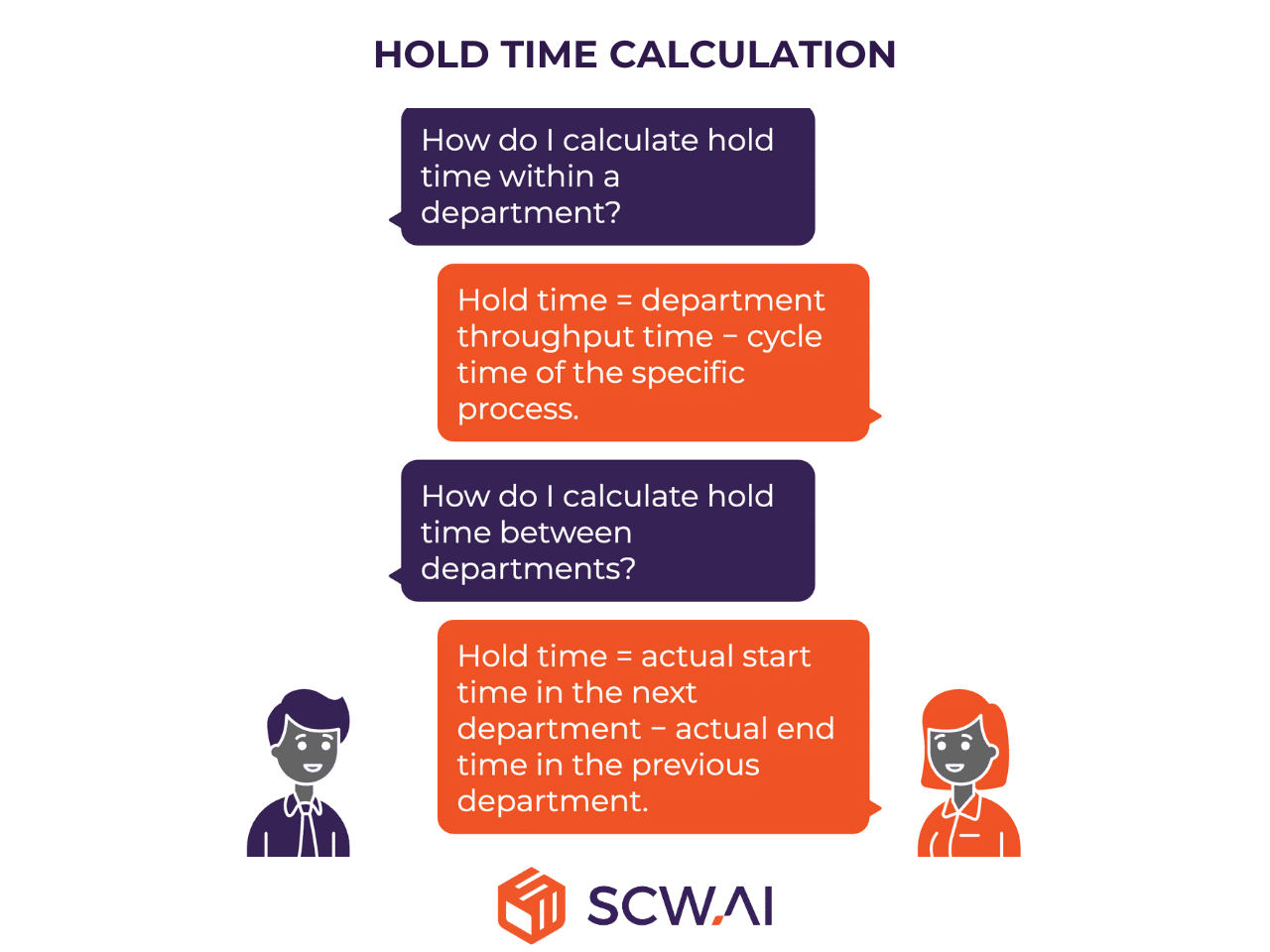
Hold time calculations depend on where the material is held:
- Within a department: Hold time = department throughput time − cycle time of the specific process.
- Between departments: Hold time = actual start time in the next department − actual end time in the previous department.
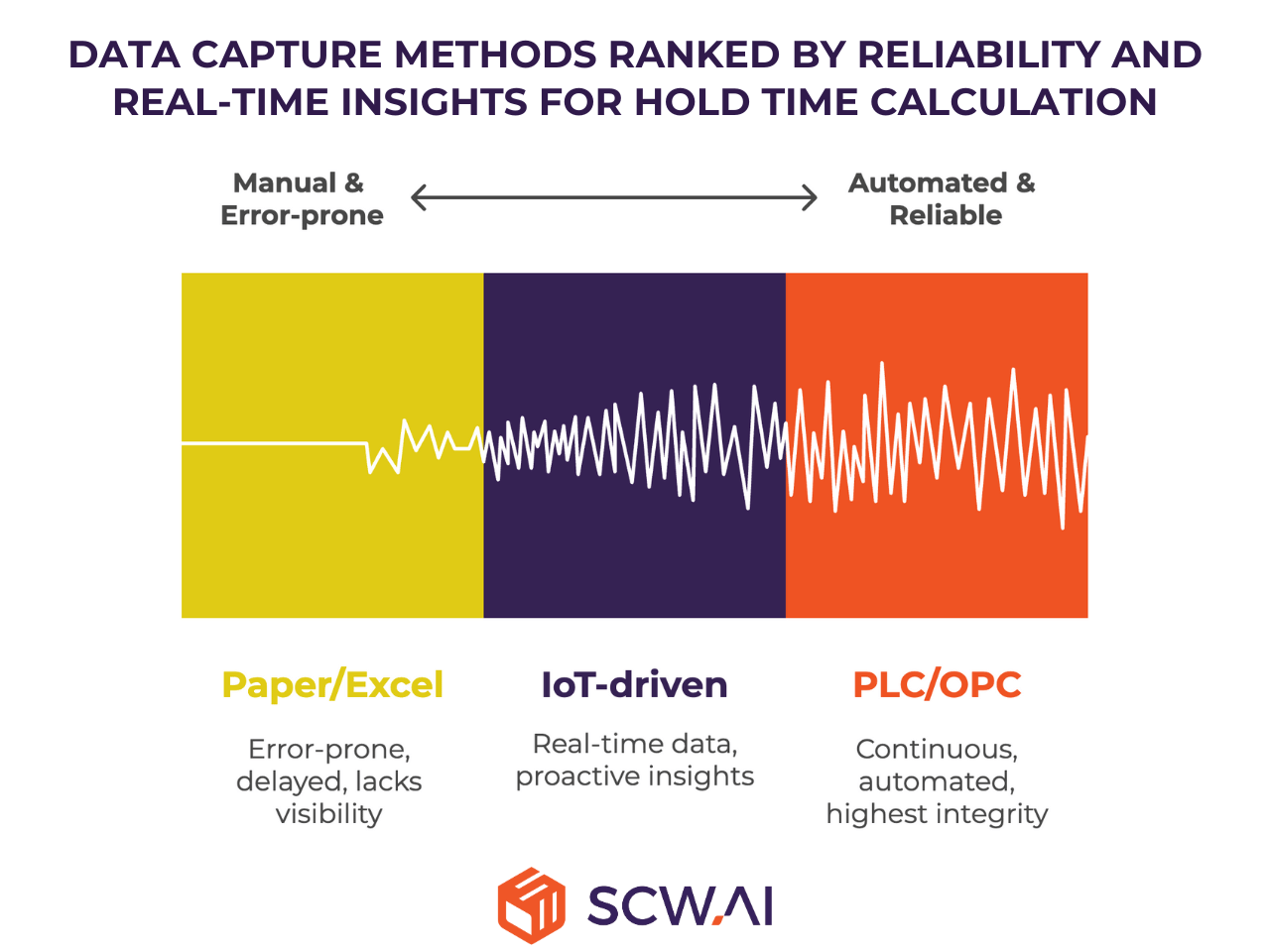
While the math is simple, the real challenge lies in accurate calculation and data capture. Methods and their reliability vary by digital maturity:
- Paper/Excel-Based Systems: Manual logging by operators. Prone to errors, delays, and missing visibility. Difficult to detect inefficiencies or quality risks early.
- IoT-driven Data Capture: Sensors on equipment record throughput, cycle times, and product counts in real-time. Data is converted into analytics dashboards, providing reliability and proactive insights.
- PLC/OPC Connections: Direct integration with equipment controls for continuous, automated data collection. Delivers the highest data integrity and real-time monitoring.
Categorizing Hold Time: From Intermediate to Bulk
Intermediate Product Hold Time
Defined for materials that have undergone one or more steps but are not yet in their final form—for example, a tablet core before coating or a granulated blend before compression. At this stage, materials are especially vulnerable to chemical shifts or microbial contamination, making validation essential.
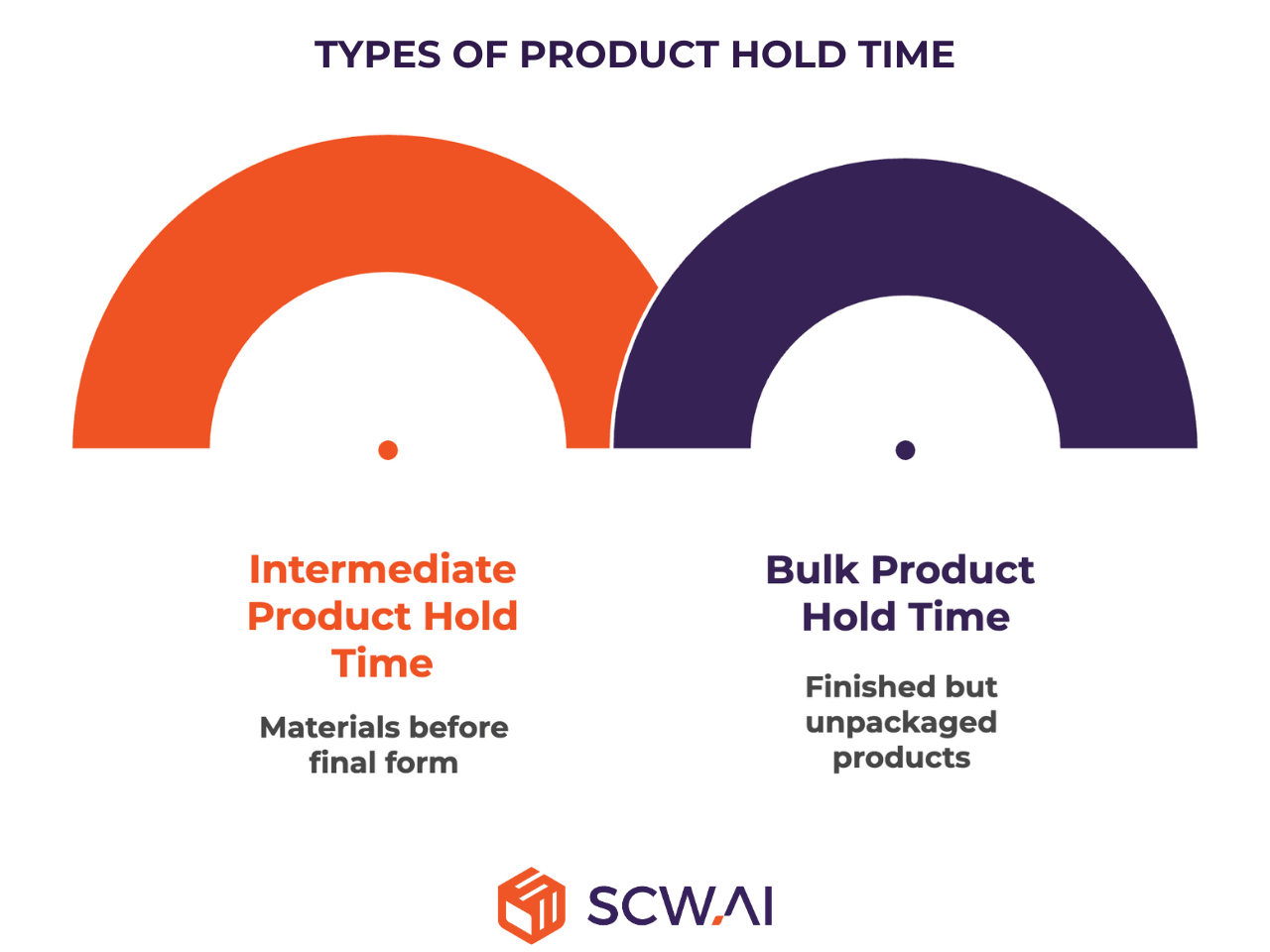
Bulk Product Hold Time
Applies to finished but unpackaged products, such as tablets or capsules stored in containers before primary packaging. This validation ensures the product retains all critical quality attributes (CQAs) during storage, protecting stability and therapeutic performance until packaging.
3 Reasons Why Optimized Hold Time Drives Business Value
By optimizing both quality-driven and operational hold times, manufacturing leaders can unlock significant gains in compliance, efficiency, and profitability.
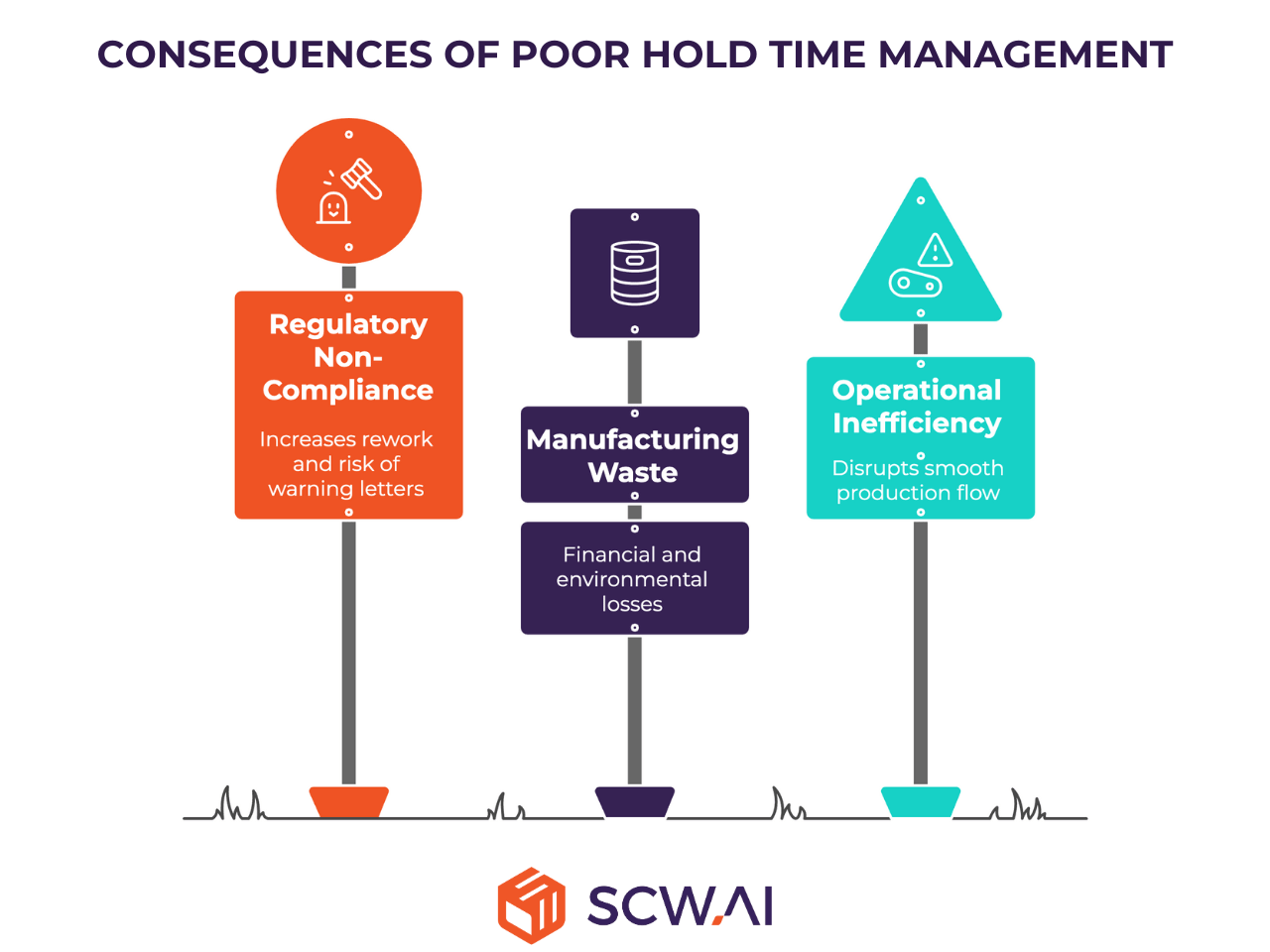
Meeting Regulatory Requirements
In a regulated industry, adherence to validated hold time limits is non-negotiable. Deviations can trigger:
- Non-conformances and batch rejections that drive up rework and cost.
- Regulatory actions such as warning letters, import bans, or recalls.
- Reputational damage, reducing customer trust and jeopardizing partnerships.
Reducing Manufacturing Waste
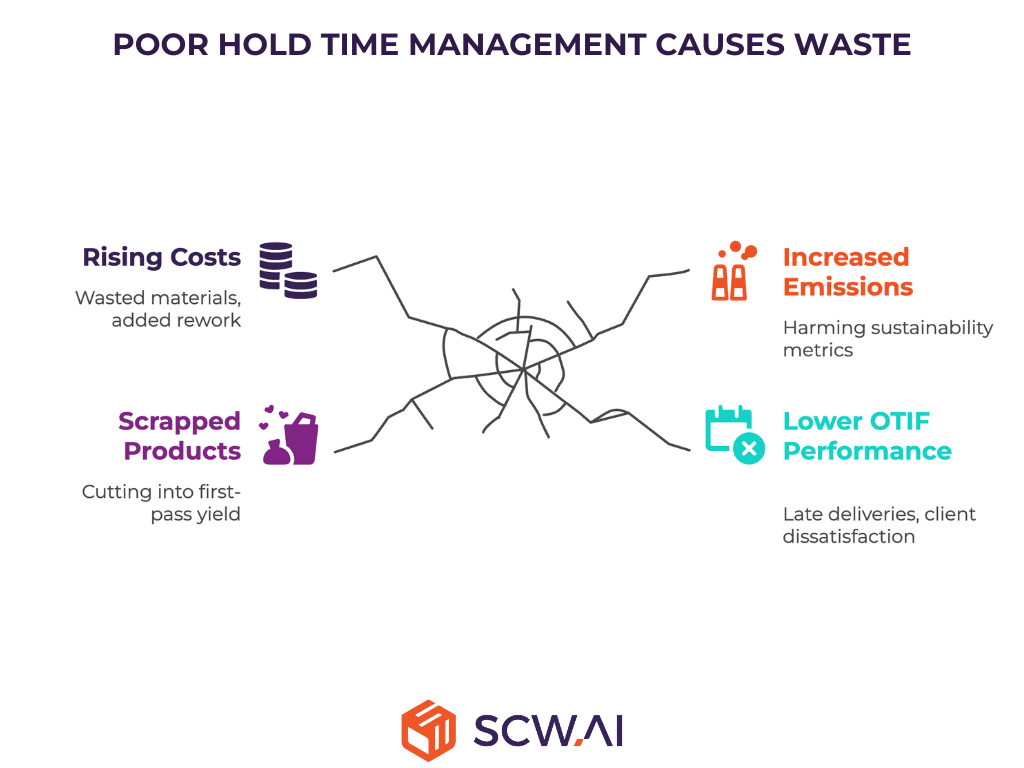
Poor hold time management creates significant financial and environmental losses for pharma companies. When materials approach or exceed their validated hold time expiration, the fallout includes:
- Scrapped intermediates or bulk products, cutting into first-pass yield.
- Rising production costs from wasted raw materials and added rework.
- Increased greenhouse gas (GHG) emissions and reliance on virgin materials, harming sustainability metrics.
- Lower On-Time, In-Full performance, leading to late deliveries, client dissatisfaction, and even financial penalties for CDMOs.
Achieving Operational Excellence and Lean Manufacturing
Operational hold times—those caused by inefficiencies rather than quality requirements—are a hidden cost in many manufacturing facilities. They represent unnecessary idle time for equipment and personnel and disrupt the smooth flow of production, directly revealing a fundamental weakness in a company’s lean manufacturing approach.
These non-value-added waiting periods are a classic form of waste (Muda) in a lean context, and addressing them is essential for improving overall performance.
Such inefficiencies have a cascading negative effect on a variety of manufacturing KPIs, highlighting where productivity and profitability are being compromised:
- Production Cost per Unit
- OEE
- Total Effective Equipment Performance (TEEP)
- Schedule Compliance
- On-Time In-Full (OTIF)
- Takt Time
- Percentage Overtime Labor Hours
To learn detailed information on more than 60 manufacturing KPIs, including the ones mentioned above, along with their calculation methods and how they’re utilized on the shop floor, we recommend downloading our free 60+ Manufacturing KPIs Handbook.
Embracing the Digital Revolution: Automated Solutions for Hold Time Management
A digital approach transforms hold time from a potential liability into a strategic advantage, ensuring both quality and efficiency.
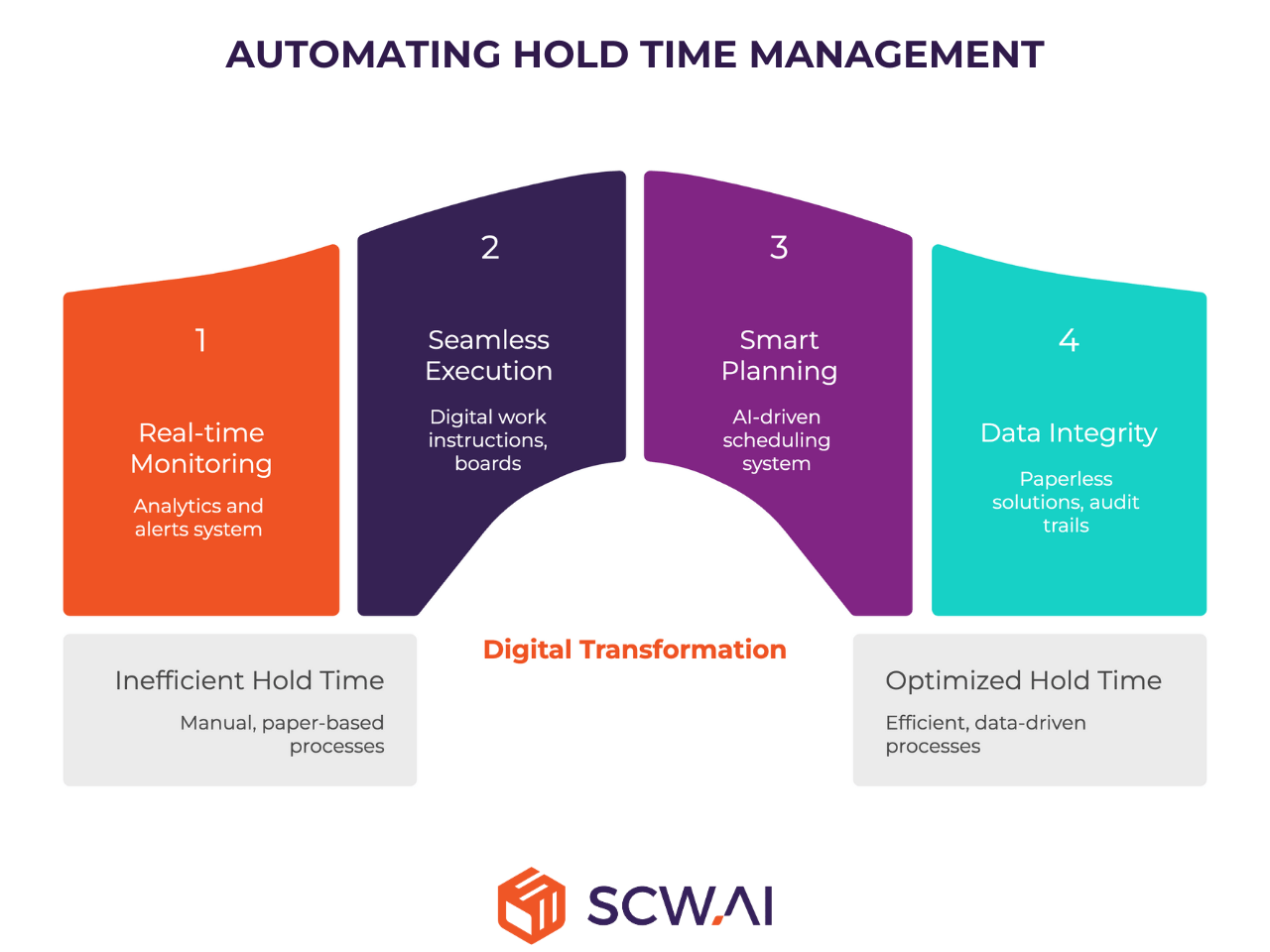
1. Real-time Hold Time Monitoring with Analytics and Alerts
Thanks to automated hold time monitoring, teams can:
- Prioritize: Proactively schedule and fast-track batches at risk of expiring.
- Prevent: Intervene before a material’s quality is compromised, avoiding costly rework or total batch loss.
- Analyze: Use historical data to identify trends and root causes of hold time issues, enabling continuous process improvement to reduce future waste.
By leveraging OPC connections and IoT sensors, manufacturers can ensure a reliable, real-time data flow into their analytics platforms. These analytics then calculate and present hold times for each work order, allowing line leaders and executives to detect performance issues and prioritize batches at risk of expiring.
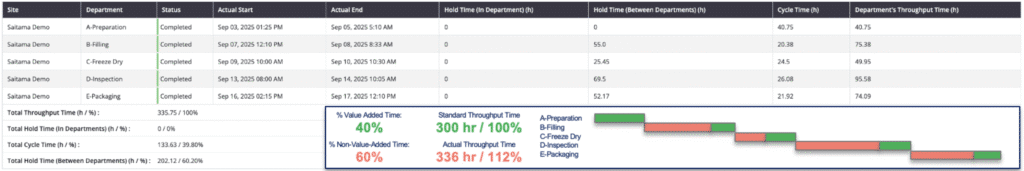
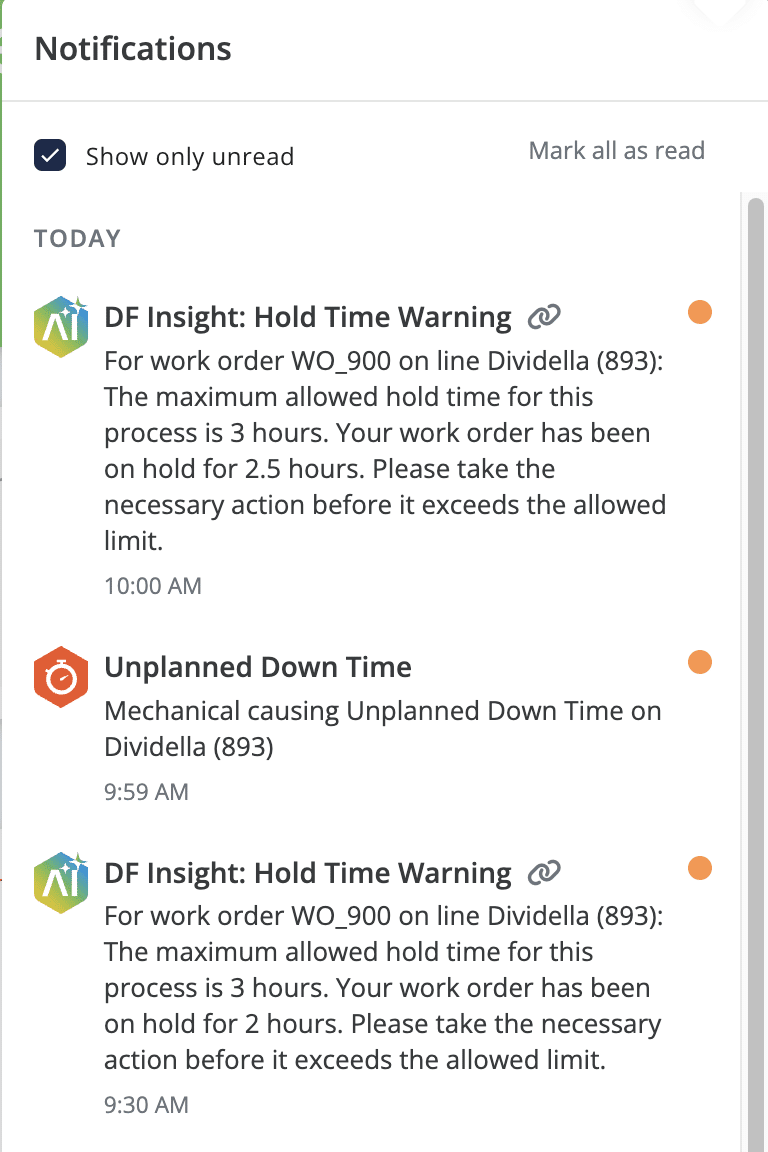
To further empower teams, manufacturers can enrich their master data with quality-related hold time thresholds for each production step. These thresholds can be used to create a color-driven verification system. For example, if a pharmaceutical manufacturer determines that the hold time between granulation and compression should be a minimum of two hours but no more than three, the system can provide a clear status.
A process at two and a half hours will appear green, highlighting that it is ready for the next step. However, a process exceeding three hours will turn red, immediately signaling to executives that they must take action to prevent quality compromise.
More sophisticated alert systems can be programmed to notify workers before the maximum hold time is reached. These alerts can even be integrated with AI production insights to support pharmaceutical companies in achieving operational excellence.
2. Seamless Executions with Digital Work Instructions & Boards
Monitoring provides the insights, but without effective execution, its positive impact on hold time management is limited. Traditional paper-based systems, such as Standard Operating Procedures (SOPs), as well as physical SQCDP and Kamishibai Boards, are often ineffective. They limit standardization, reduce accountability, and constrain data-driven tier meetings, ultimately hampering lean management principles.
2.1. Digital Work Instructions
Digital work instructions provide dynamic, user-friendly guidance. By integrating videos, images, and checklists, these instructions improve standardization, ensuring that quality-related hold times are consistently respected.
In addition, people work more effectively and efficiently thanks to standardization provided by digital work instructions, which directly contributes to a reduction in operational hold times.
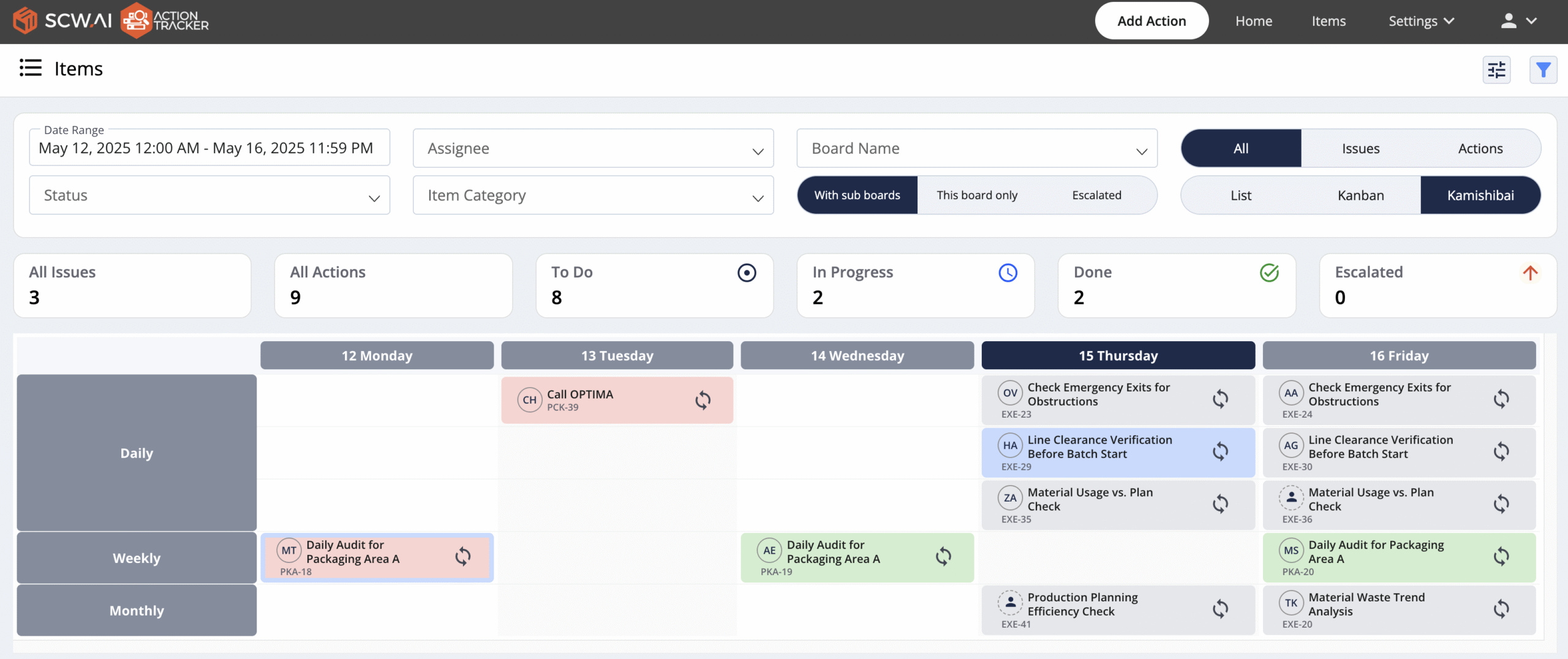
2.2. Digital Kamishibai Boards
Digital Kamishibai Boards enhance accountability and ease task allocation by clearly defining which worker is responsible for a task and its due date. It is also possible to attach relevant digital work instructions directly to these tasks, ensuring that every team member has the resources needed to perform their job correctly. This level of clarity and integration simplifies task management and ensures everyone is aligned on their responsibilities.
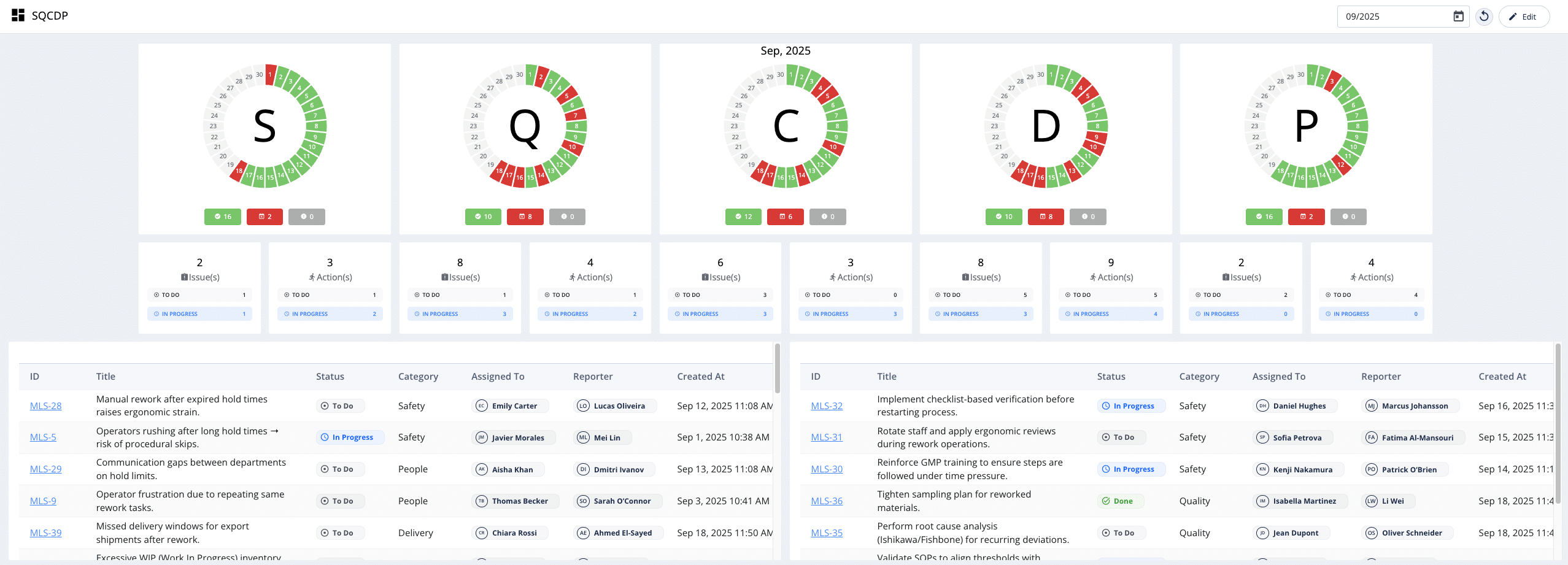
2.3. Digital SQCDP Boards
Digital SQCDP (Safety, Quality, Cost, Delivery, People) boards are designed to make shop floor tier meetings genuinely data-driven. By providing crucial, real-time data on these key metrics, digital boards allow teams to focus on actual issues rather than spending time on data collection. Frequent problems with hold times, for example, will inevitably cause the quality, cost, and delivery components to turn red during monthly checks. This prompts the management team to immediately assign supervisors to investigate the root cause and prepare Ishikawa Fishbone Diagrams, accelerating the path to problem resolution.
To learn more about Action Tracker, which includes solutions such as Digital Work Instructions, the Digital Kamishibai Board, and the Digital SQCDP Board, click the button below.
3. Smart Planning: Driving Quality by Design (QbD)
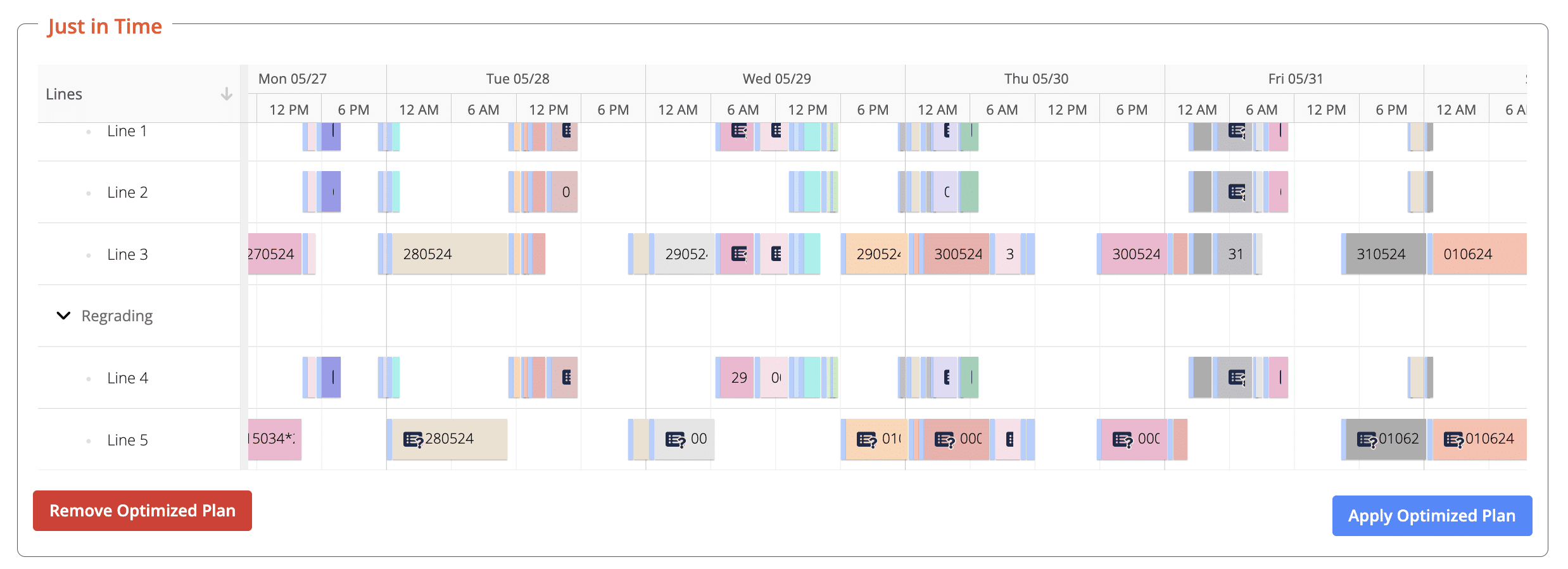
With AI-driven Advanced Planning and Scheduling (APS) systems, hold time constraints can be embedded directly into production planning. Instead of reacting to delays or deviations, schedules are built to respect validated hold times from the start.
Benefits include:
- Optimized production schedules with time buffers that account for required hold times.
- Visualized schedules highlighting where holds occur and ensuring visibility across teams.
- Seamless alignment of planning and compliance, reducing the likelihood of deviations.
This proactive approach reflects the Quality by Design (QbD) principle: quality is not inspected at the end but designed into the process from the beginning.
4. Ensuring Data Integrity and Audit Readiness with Paperless Solutions
Pharma specific paperless manufacturing solutions strengthen compliance and streamline workflows by:
- Creating Immutable Audit Trails: All actions and timestamps are logged automatically.
- Enhancing Security: Features like e-signatures and automatic version control ensure accountability and compliance with regulatory standards such as cGMP and ALCOA+.
- Accelerating Documentation: Digital logbooks and batch records reduce documentation time by up to 85%, while also minimizing transcription errors.
- Streamlining Audits: Data is centralized, searchable, and instantly accessible—allowing teams to respond confidently to inspections.
Automated alerts for out-of-specification events further ensure that deviations are not only detected but also documented with corrective actions, protecting compliance and improving quality maturity management.
Optimize Your Production: Partner with SCW.AI
SCW.AI’s Digital Factory Platform is purpose-built for the pharmaceutical industry, offering an integrated solution to the complexities of modern manufacturing. By combining real-time monitoring, seamless execution, intelligent scheduling, and paperless quality systems, it provides a powerful framework for effective hold time management.
The impact extends far beyond hold times. Our platform helps:
- Minimize changeovers and unplanned downtime.
- Boost critical KPIs such as OEE, FPY, and OTIF.
- Increase revenue and reduce costs through smarter, leaner operations.
To learn more about process hold time, or our Digital Factory Platform, and its pricing, you can contact us.
To truly witness the platform in action, engage with our professionals to discuss pricing, implementation, and potential ROI by booking a demo with us.