Pharma 4.0 is set to revolutionize the pharmaceutical industry, offering substantial improvements in efficiency, quality, and innovation. According to WEF, Johnson & Johnson, for instance, has seen a:
- 50% reduction in unplanned downtime
- 4.5 percentage point increase in OTIF scores,
- And 90% decrease in product development testing lead times due to pharma 4.0 investments.
Despite these benefits, pharma 4.0 adoption remains low. Mike Walker, Executive Director of Global Healthcare and Life Sciences Digital Strategy of Microsoft notes that only 1% to 3% of pharmaceutical companies qualify as Pharma 4.0 facilities (See video below). Barriers include stringent regulations, data security concerns, and high legacy system costs. However, modern technologies and management practices can overcome these challenges.
In this article, we will explore Pharma 4.0 in depth, examining its benefits, key technologies, and strategies for successful implementation. Through real-world examples and practical insights, we aim to equip pharmaceutical managers with the knowledge and tools necessary to modernize their factories, optimize operations, and stay competitive in a rapidly evolving industry.
What is Pharma 4.0?
Pharma 4.0, a framework developed by the International Society for Pharmaceutical Engineering (ISPE), modernizes pharmaceutical manufacturing by integrating advanced digital technologies. It is an extension of the Industry 4.0 concept, created specifically for sector’s unique needs. While Industry 4.0 broadly addresses advancements in manufacturing through automation, data analysis, and advanced technologies, Pharma 4.0 covers the distinct challenges faced in pharma manufacturing. For instance, unlike heavy production industries where machine faults are common, the pharma industry mostly deals with lengthy changeover times, meticulous cleanups and setups.
Pharma 4.0 significantly improves key manufacturing metrics, such as production efficiency, quality control, and time-to-market. By automating processes and utilizing advanced analytics, companies can enhance overall equipment effectiveness (OEE), ensure line balancing, and streamline work order management to achieve production excellence.
A major advancement within Pharma 4.0 is the adoption of paperless manufacturing solutions. Traditional logbooks and batch records are replaced with electronic batch records and digital logbooks, enhancing data compliance with ALCOA+ data integrity principles, reducing human error, and time needed for reporting.
Pharma 4.0 also aligns digitalization with lean manufacturing practices, creating more efficient and agile production environments. Digital tools enable better resource management, minimize waste, and optimize workflows, while AI/ML models support predictive maintenance, job shop scheduling, and process optimization. This intelligent automation enhances flexibility and empowers managers to make optimal decisions quickly.
Why is Pharma 4.0 Important Now?
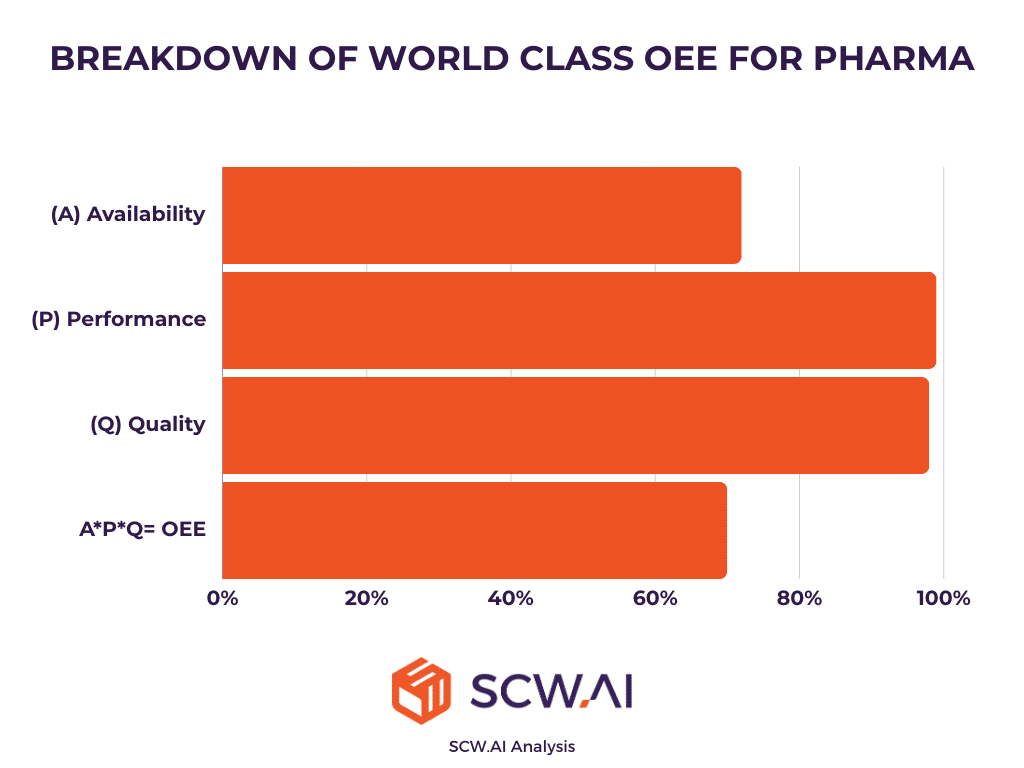
According to our OEE benchmarking for the pharmaceutical industry, a typical manufacturer has an OEE of around 37, with nearly 50% of losses occurring due to planned and unplanned downtimes. An additional 20% of losses are attributed to performance issues, such as micro-stops, and 6% are due to quality losses (See image below at the left).
In contrast, our analysis shows that world-class OEE for pharmaceutical manufacturers is 70%. These top-performing factories have augmented and automated their processes with pharma 4.0 technologies. For a typical pharma 4.0 manufacturer, availability is around 75%, and performance and quality losses approach 0% (See image below at right).

Improving OEE from 37 to 70 offers several significant benefits for manufacturers.
- According to our ROI calculator, such an increase can improve annual returns by $15M to $21M for a factory operating 16 hours per day with 50 lines and an initial revenue of $50M, depending on how the improved productivity is allocated between capacity and cost reduction.
- A reduction in quality losses decreases the risk of receiving warning letters or penalties from organizations like the FDA.
- Additionally, higher OEE, associated with less waste, enhances the sustainability KPIs of manufacturers.
These improvements are crucial for various types of pharmaceutical manufacturers. For instance, cost reduction and productivity improvement are vital for the competitive generic pharmaceutical industry. Meanwhile, McKinsey reports that sterile pharma manufacturers are facing a surge in demand post-COVID-19. The report specifically advises these manufacturers to invest in improving OEE through digitalization, which requires less time to yield results compared to opening new facilities to improve production capacity.
Orphan drug producers also benefit from the higher quality of their products. In summary, Pharma 4.0 investments are essential for all companies operating in the life sciences industry.
Categories and Technologies of Pharma 4.0 Solutions
Pharma 4.0 technologies address various business needs of factories. As shown in the figure below, these solutions can be categorized into five distinct groups.
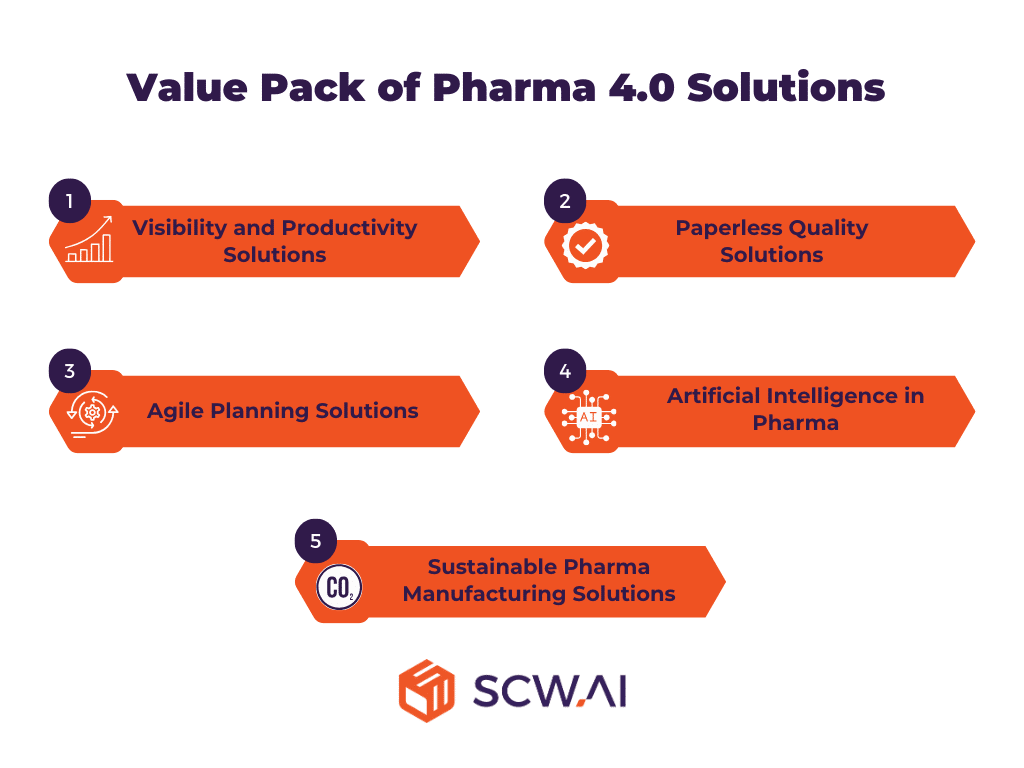
1. Visibility and Productivity Solutions for Pharma Manufacturers
Automated data collection and representation on live dashboards are the cornerstones of issue detection and root cause analysis. Additionally, dashboards and manufacturing apps enable managers to monitor progress towards goals effectively.
To ensure shop floor visibility, pharmaceutical manufacturers must first collect real-time granular data about machines, production lines, and labor. Various technologies facilitate this process. For example, OPC connections and Modbus PLCs can automate the collection of line and machine data. However, many pharmaceutical manufacturers lack machines with these capabilities or are restricted by regulations and data protection concerns from sharing OPC/PLC data. In such cases, IoT automation can help collect real-time shop floor data. Furthermore, IoT devices enable manufacturers to gather labor data as well.
As a second step, pharmaceutical manufacturers need to display real-time data on dashboards to identify business opportunities and bottlenecks. A wide range of dashboards can be designed to showcase key manufacturing KPIs crucial for production, such as OEE, takt time, labor variance for the cleanup process, and more. Additionally, real-time data can be programmed to trigger alerts. For instance, if an unplanned downtime occurs on a specific line, a notification can be sent to the line leader and executives.
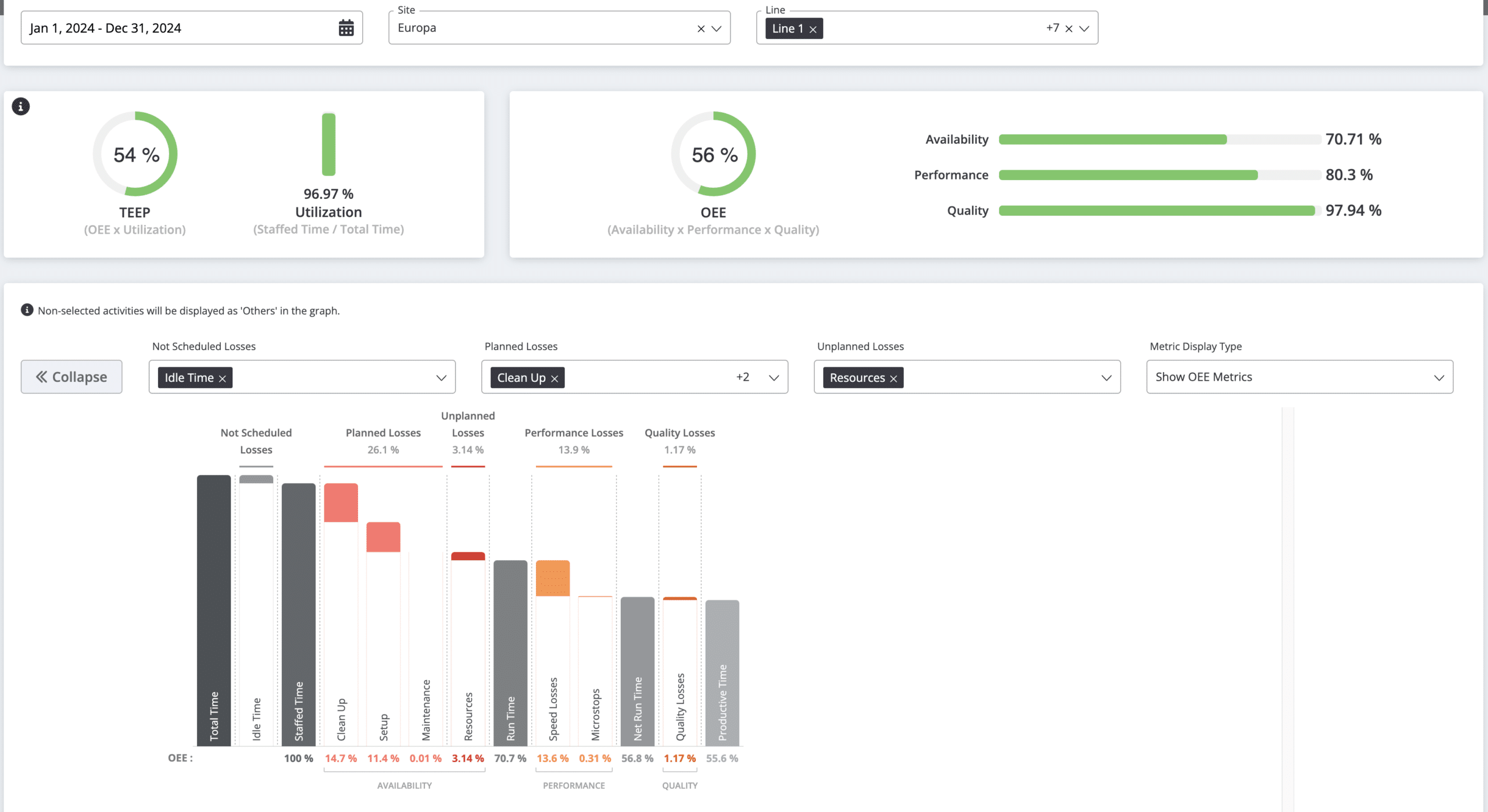
It is advisable to design a mobile application for displaying reports and sending notifications to personnel. By utilizing manufacturing apps, the leadership team can monitor production from anywhere at any time. This flexibility allows pharmaceutical manufacturers to adopt a more dynamic working style, which is increasingly demanded by engineers. Consequently, offering hybrid working options for the management team can help address the labor shortage crisis facing manufacturers.
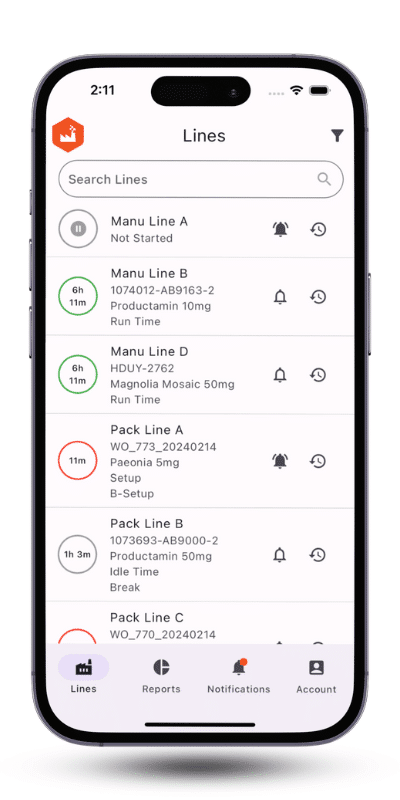

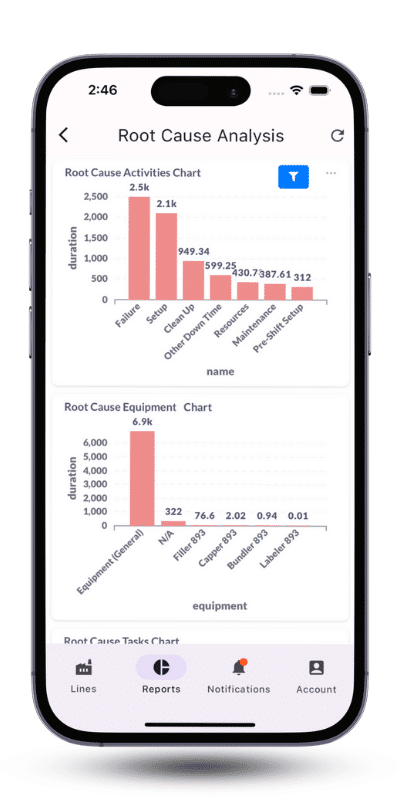
By investing in visibility and productivity through Pharma 4.0 solutions, one of our sterile pharma clients identified the bottlenecks causing planned and unplanned downtimes. By actively monitoring their lines, they were able to reduce downtime by 30%. To learn more, read our sterile pharma CDMO case study.
2. Paperless Quality Solutions for Pharma Manufacturers
To comply with cGMP regulations, pharmaceutical manufacturers must record every phase of production in logbooks and batch records, and present them to auditors during investigations. The FDA and similar governmental bodies require that these production records adhere to ALCOA+ data integrity principles, which stipulate that:
- Records must be attributable to specific users.
- Each record must be traceable via signatures.
- Data must be stored in an audit-ready format.
- Archiving must ensure that all past recordings are securely stored.
As Indranil Nandi, Chief Scientific Officer at Jubilant, mentions in the video below, managing thousands of log and batch entries annually is a time-consuming and error-prone task without paperless manufacturing solutions.
Pharma 4.0 solutions address this specific industry need through Digital Logbooks and Digital Batch Records. These paperless quality solutions are designed to comply with ALCOA+ data integrity principles, storing data in secure databases that are always audit-ready. Features such as out-of-specification notifications, mandatory data entry enforcement, e-signatures, and more ensure compliance with FDA 21 CFR Part 11.
The result is a significant reduction in the probability of receiving warning letters. For instance, in 2023, around 8% of all FDA warning letters were mitigated by using Digital Logbooks and Batch Records.
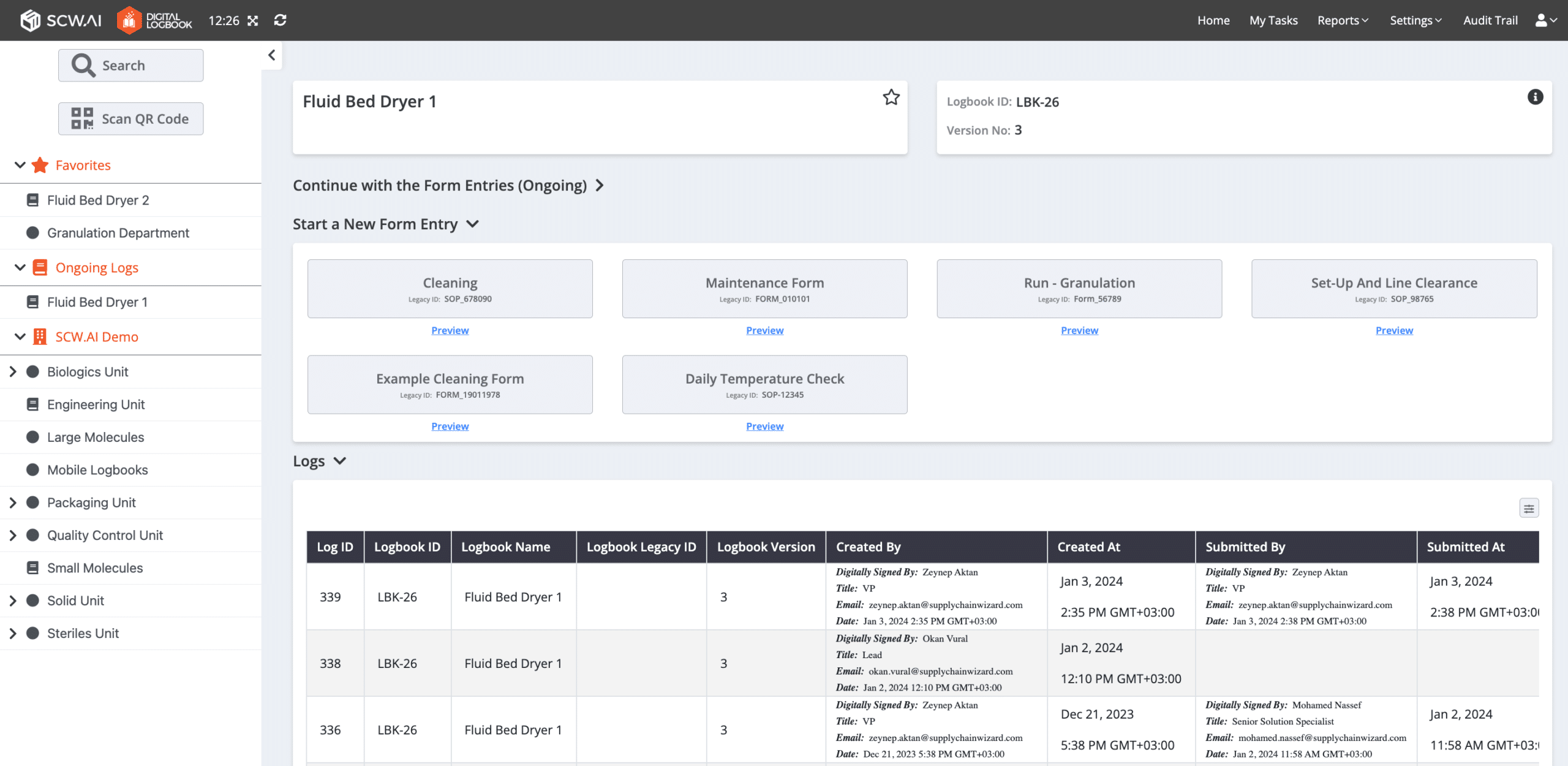
In addition to improved compliance, paperless quality solutions can reduce data entry time by up to 85%, according to our case studies. The main reasons for this improvement are automatic calculations, data exchange with other digital solutions, and a user-friendly digital interface. For instance, one of our customers reported that archiving a logbook now takes only 12 clicks, as opposed to days of manual processing.
3. Agile Planning Solutions
Scheduling and planning activities, as well as monitoring their success, are crucial for assessing pharmaceutical companies’ performance in:
- Cycle times
- Labor productivity
- Total productive maintenance practices, and more.
Pharma 4.0 solutions address these needs effectively. For instance, advanced planning and scheduling (APS) systems allow manufacturers to assign work orders to specific lines. As we will illustrate further in the AI in pharma section, APS tools can be enhanced with AI algorithms to optimize schedules for various business scenarios. Additionally, there are digital solutions for labor scheduling that provide a user-friendly interface for managing these activities. Manufacturers can integrate labor skill matrices with schedulers to assign operators based on their skills and experience.
Planning managers can also use schedule adherence reports to determine whether targets and actual durations for specific activities align. These reports offer granular visualization of schedule adherence, which can be highly beneficial for pharmaceutical companies. For example, one of our pharma clients reduced changeover variance by half by utilizing detailed custom reports on changeover variance.
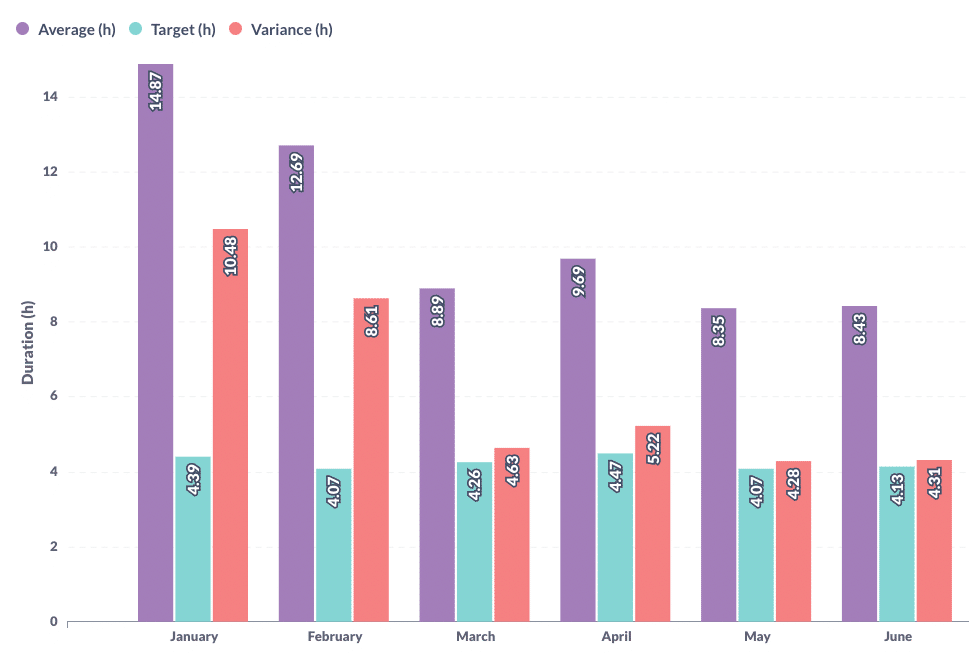
Maintenance activities can also be monitored and optimized through Maintenance Performance Reports, which provide real-time data on metrics such as mean time to failure, mean time between failures, and mean time to repair, among others. As we will discuss shortly, this data can be used to build machine learning models for predictive maintenance.
4. Artificial Intelligence in Pharma
AI enhances intelligent automation in the pharmaceutical industry by facilitating automated decision-making and/or augmenting the decision-making process for management teams. As shown in the image below, the use of AI in pharma can be categorized into four different areas.
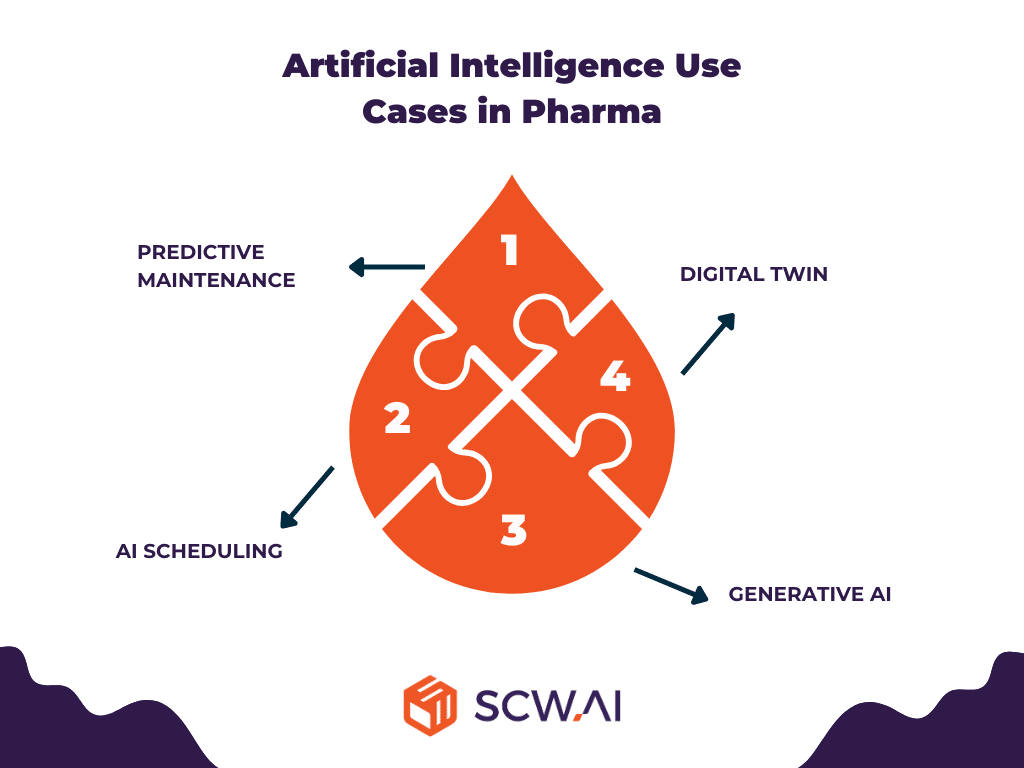
4.1 Predictive Maintenance in Pharma Manufacturing
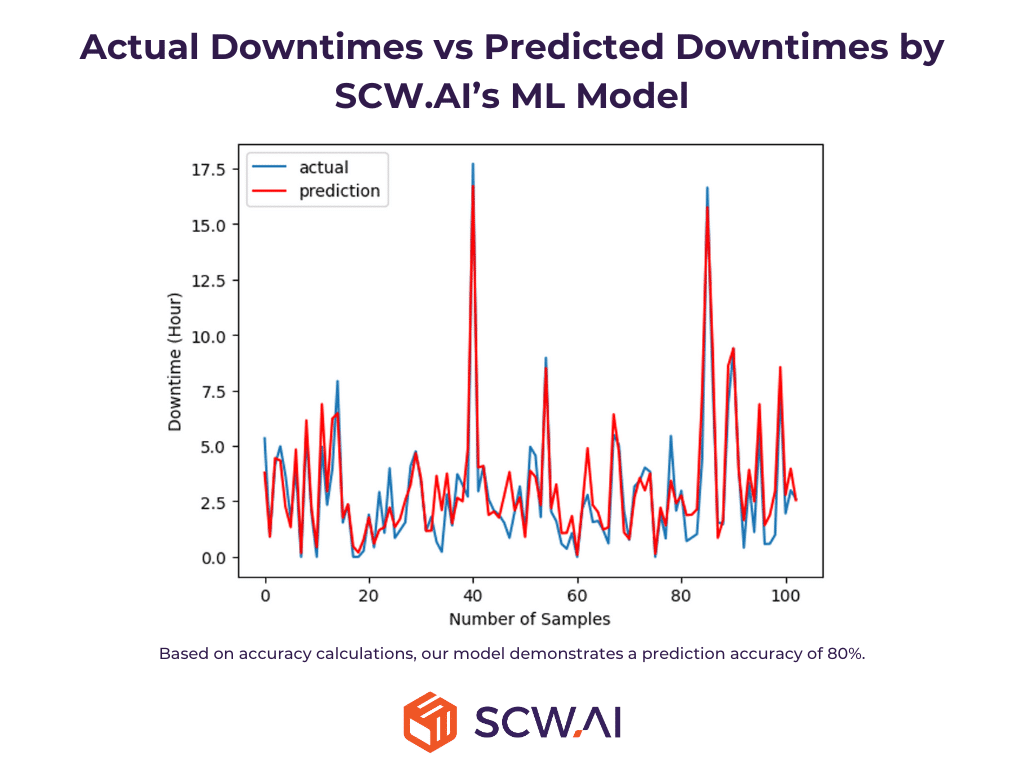
Predictive maintenance involves forecasting machine failure times using advanced analytics to schedule maintenance before unplanned downtime occurs. Unlike preventive maintenance, which schedules regular maintenance regardless of actual machine condition, predictive maintenance optimizes maintenance scheduling to minimize unplanned downtime without significantly increasing planned maintenance time. Thus, as highlighted by Deloitte, predictive maintenance can reduce maintenance costs by 10% while decreasing downtime. For instance, Johnson and Johnson reduced machine downtime 50% with ML driven predictive maintenance.
The image below illustrates how SCW.AI’s predictive maintenance model statistically significantly predicts real machine failures.
4.2 AI Scheduling in Pharma Manufacturing
When it comes to pharmaceutical manufacturing, two scheduling optimization scenarios can be beneficial:
- Changeover Minimization: Due to regulations, line clearance is vital for pharma manufacturers. The cleaning period can be minimized by scheduling products that require similar raw materials consecutively.
- Just-in-Time Delivery: For some drugs, such as radiopharmaceuticals have very short lifespans. For instance, the lifespan of some radioactive isotopes is sometimes measured in hours. In these cases, just-in-time delivery becomes essential, as further explained in the video below.
Other scheduling optimizations, such as OTIF maximization, makespan minimization, and energy consumption minimization, can be crucial for your business.
AI schedulers are effective tools for addressing various scheduling optimization scenarios in pharmaceutical manufacturing. As illustrated in the image below, SCW.AI’s scheduler algorithms, for instance, optimize job shop scheduling for different business needs.
4.3 Generative AI in Pharma Manufacturing
Generative AI can be an effective tool for pharmaceutical companies to improve production quality, efficiency, and compliance through training. By uploading regulations and SOPs into a generative AI model, pharmaceutical manufacturers can educate the AI about the specific details and rules of the business.
Based on this input, generative AI can create tailored educational content for workers based on their roles. It can design various learning materials, such as readings, videos, and images. Additionally, by creating quizzes, the AI can assess which employees have successfully completed the training and which ones need further education.
4.4 Digital Twin in Pharma Manufacturing
The realistic simulation capability of digital twins is another beneficial AI use case for pharmaceutical manufacturers. Engineers can use a digital replica of the factory to run production with different parameter values and observe the impact on production speed, quality, cost, machine health, and more. This allows manufacturers to continuously optimize their batches, detect machine degradation, and accurately predict the future state of their factory.
For instance, Dr. Reddy’s Laboratories reduced production costs by 21% by utilizing digital twin technology.
Unlock the power of AI in pharma. Download our free white paper, “AI in Pharma: Use Cases, Success Stories & Challenges” to explore real-world applications and the benefits top manufacturers have achieved.

5. Sustainable Pharma Manufacturing Solutions
With existing regulations such as CSRD and anticipated upcoming sustainability regulations, green transformation has become a crucial agenda for pharmaceutical companies. Specific Pharma 4.0 tools have emerged to address this industry need.
While OEE improvements and paperless quality solutions help reduce environmental impact, carbon accounting is a major part of the solution. Carbon trackers provide granular data displayed on real-time dashboards, showing which activities emit how much CO2.
Additionally, some sustainability software offers similar data visualization for other environmental metrics, such as water consumption and virgin material usage. This gives the management team complete visibility into the environmental costs of their business, enabling them to take data-driven actions to minimize externalities.
Importance of Strategic Partnerships with Technology Vendors for Pharma 4.0 Investments
In our experience, many pharmaceutical companies collaborate with technology vendors to outsource their pharma 4.0 solutions. This trend is unsurprising, as many manufacturers face resource constraints for in-house development. However, selecting the right technology vendor remains a significant challenge for pharma managers.
The ideal technology vendor varies for each company. Here are some 3 common factors to consider while choosing a partner:
1. Industry Focus
A lot of technology vendors tend to specialize in specific manufacturing industries. This inclination influences the solutions they offer, as they design products with a focus on addressing the unique challenges of particular industries. Therefore, it is advisable to collaborate with a tech firm that knows the unique challenges of the pharma industry and designs products accordingly.
2. Cloud vs. Low-code Solutions
Cloud manufacturing solutions may be more suitable for pharma organizations that seek easily scalable solutions and lack a dedicated IT/developer team or a robust IT infrastructure, which is often the case for typical pharmaceutical manufacturers. The industry has lagged behind in digitalization, resulting in chronic issues of visibility, productivity, and agility that need prompt addressing. Moreover, the manufacturing industry, in general, faces challenges in hiring skilled developers. Consequently, deploying and maintaining a low-code solution may not be feasible for many pharma manufacturers. With cloud solutions, you can leverage a technology vendor’s digital solution through an internet connection much like logging into a social media account.
3. Quality of Customer Success Team of Vendors
Effectively utilizing digital solutions can pose a challenge for employees, potentially resulting in the partial realization of expected business benefits. To fully grasp the advantages of digitalization, the quality of the Customer Success Team is crucial. This team guides your workers in navigating the intricacies of digital tools, ultimately enhancing ROI. It is a well-known fact that many technology vendors lack such qualities or may impose additional high fees on customers. Therefore, it is advisable to thoroughly examine the customer support policies of technology vendors before entering into a partnership.
Become a Pharma 4.0 with SCW.AI
SCW.AI delivers comprehensive Pharma 4.0 solutions tailored to the unique needs of the pharmaceutical industry. Our Digital Factory Platform empowers you to transform every aspect of your facility, offering:
- Enhanced Visibility and Productivity: Leverage IoT devices and real-time monitoring with support for existing PLC-OPC integrations if they exist. Gain valuable insights with our OEE Tracker, Labor Tracker, and Asset Tracker. Plus, benefit from remote production monitoring with our mobile manufacturing app.
- Paperless Quality Management: Ensure GMP compliance with our intuitive Digital Logbook, designed specifically for the pharmaceutical industry.
- Agile Planning for Efficiency: Optimize your operations with our Maintenance Performance Reports, OTIF Reports, Schedule Adherence Reports, and more.
- AI-Powered Optimization: Unlock significant efficiency gains with our predictive maintenance and scheduling algorithms. Additionally, we offer digital twin technology and generative AI models for a variety of use cases.
If you have further questions about the concept of pharma 4.0 and how we can support you for transformation, contact us.
Ready to unlock the full potential of Pharma 4.0? Book a demo today to explore our value package and the capabilities of the Digital Factory Platform.